1. Background
In industrial societies, reduced physical activity is the main reason for the prevalence of obesity, hypertension, and cardiovascular diseases (1). Obesity induces severe premature cardiac aging, cardiac lipoapoptosis, obesity-associated cardiac dysfunction, and left ventricular hypertrophy (2-4). The PI3K-Akt pathway in upstream signaling of Insulin Grows Factors (IGFs), insulin, and insulin receptor substrate (IRS) regulates forkhead box protein O1 (FOXO1) transcriptional activity through phosphorylating FOXO1 (5). Phosphorylated FOXO1 is excluded from the nucleus and prevents muscle atrophy. FOXO1, a member of the forkhead transcription factor protein, is predominantly expressed in most muscle types such as cardiac and skeletal muscles (5, 6). FOX proteins are a family of transcription factors that play important roles in regulating the expression of genes involved in cell growth, proliferation, differentiation, and longevity. FOXO1 is a key regulator of muscle cell proliferation, differentiation, and metabolism (7). Additionally, it plays a role in muscle atrophy by activating the ubiquitin-proteasome proteolysis pathway (8, 9).
Several studies have shown that the physical training-mediated regulation of MuRF1, MAFbx, and FOXO1 gene expression depends on the intensity, type, and history of training. Interestingly, FOXO1 inhibitors can prevent cardiac atrophy (10-13). Indeed, FOXO1 suppresses muscle degeneration via proliferator‐activated receptor gamma co-activator 1‐alpha (PGC-1α) as a mitochondrial biogenesis cofactor (10, 14, 15). PGC-1α as a direct co-activator of FOXO1 and indirect co-activator of Peroxisome proliferator-activated receptor γ (PPARγ) is a metabolic transcriptional factor required for oxidative metabolism, mitochondrial biogenesis, and slow-twitch fiber formation (16, 17). Several reports showed that insulin/Akt signaling reduces the basal PGC-1α promoter activity (18). In addition, PGC-1α directly controls FOXO1, by reducing the activity of NF-ҡB signaling and inflammatory factors, which prevents muscle atrophy (19). Physical activity induces the expression of the PGC-1 gene in the skeletal muscle, which leads to the stimulation of mitochondrial biogenesis (20).
Curcumin is a phytopolylphenol pigment isolated from the plant Curcuma longa, with anti-inflammatory, antioxidant, and anti-apoptosis effects (21-24). Activating AMP-dependent protein kinase (AMPK) increases PGC-1α expression and subsequently inhibits FOXO1 (25, 26). In addition, curcumin has cardioprotective effects in patients and at-risk individuals (25).
2. Objectives
In this study, the simultaneous impact of resistance training and curcumin consumption was assessed on preventing cardiac muscle atrophy in the rat model of obesity.
3. Methods
3.1. Animal and Obesity Model
In this study, 24 eight-week-old male Sprague-Dawley rats (180 ± 20 g), purchased from the Shiraz University of Medical Sciences Animal Lab, were kept in a controlled environment (12/12h light/dark cycle, 40-60% humidity, 22°C ± 3°C). Rats received a high-fat diet consisting of 50% of the whole energy derived from soy oil, 20% protein, and 30% carbohydrate, with no food restriction, kept in polyethylene cages until they reached the ideal weight of 319 ± 30 g and were identified obese based on the Lee index (26, 27). Then, the rats were randomly divided into four groups of placebo (n=6), curcumin (n=6), resistance training (n=6), and resistance training+curcumin. The placebo group received methylcellulose in gavage to remove the effect of the gavage process on the results.
3.2. Rodent Resistance Training
First, rats were trained for resistance training on the ladder with weight lifting for 10-15 minutes for a week. In the first week, rats lifted 20% of their weight that reached 80% until the eighth week. The training was performed in three sets and repeated five times. There was a one-minute break in each set and a two-minute break between the sets. The resistance training sessions were performed twice a day with a six-hour gap, three times a week, which continued for eight weeks. The training was performed on a special one-meter ladder with an 85-degree slope that consisted of 26 stairs (11).
3.3. Curcumin Treatment Protocol
Curcumin (Sigma, Germany) was dissolved in Dimethyl Sulfoxide (DMSO, Sigma, Germany) to reach a 10% solution. Rats in the curcumin and curcumin + resistance training groups received 150 mg/kg of curcumin combined with methylcellulose daily by gavage. The effect of such a dose of curcumin on weight loss and insulin resistance has been proven in another study (28). Twenty-four hours after the last training session, rats were ethically anesthetized by intraperitoneal injection of pentobarbital (150 mg/kg, Abidi Company, Iran), and then the hearts were quickly harvested. The heart weight was measured with a scale (0.001 accuracy) and then rapidly frozen and kept at -80°C until lab tests were performed (11, 27).
3.4. RNA Extraction of Heart and RT-PCR Assay
First, 50 mg of left ventricular muscle was isolated and homogenized in the RNAx buffer by a homogenizer. The total RNA was extracted using the RNXplus kit (Cinna Gen, Iran) and kept at -80°C until PCR analysis. The quantity of extracted RNA was evaluated with a spectrophotometer (Nano-Drop Technologies, Wilmington, DE, USA). The mean Optical Density (OD) of 1.92 indicated an appropriate quality of extracted RNA. The complementary DNA (cDNA) was synthesized from RNAs using RevertAid First Strand cDNA Synthesis Kit (Fermentas, USA), Reverse Transcriptase (RT) enzyme, and oligo dT primer. The RT-PCR assay was conducted using the SYBR green technique by SYBR Premix Ex Taq II (Takara, Japan) with a Lightcycler rapid thermal cycler (Corbett RG-6000, QIAGEN, Germany). Target gene primers were designed with AlleleID version 5, and their assignments were evaluated with Primer-blast. β2-microglobulin was employed as a Housekeeping Gene and internal control of PCR (26).
3.5. Statistical Analysis
The results of gene expression are the outcomes of repeating three independent tests (Mean ± SD). The first part of the statistical method related to the description of test variables was performed using descriptive statistics. For the comparison with the control group, one-way ANOVA was applied, followed by the Tukey post hoc test for identifying the differences between the two groups. All the analyses were conducted through SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). The significance level was set at 0.05.
4. Results
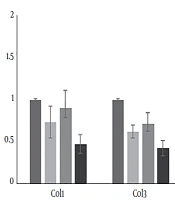
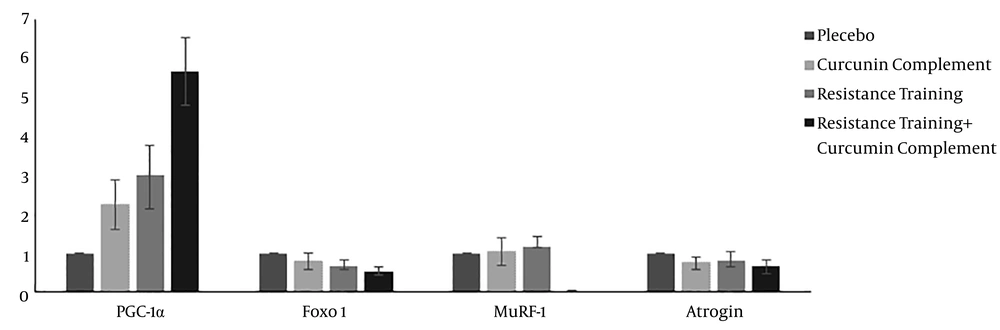
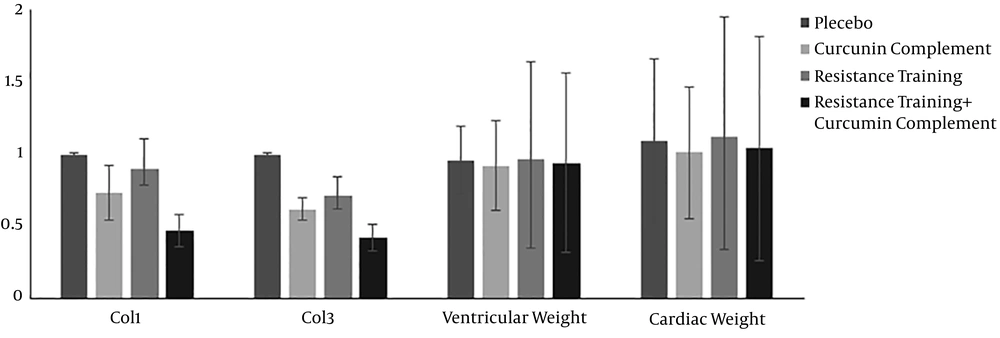
The descriptive statistics of the test variables (mean ± SD) and statistically significant differences between the groups are presented in Table 1. The mean initial and final weights of the resistance training group were 325.87 ± 5.21 and 470.06 ± 6.89 g, respectively. The mean expression levels of PGC-1α, FOXO1, MuRF-1, and Atrogin-1 are compared with the values of the placebo group, and the results are shown in Figure 1. Moreover, the mean levels of CoL1 and CoL3 expression and the weight ratio of the left ventricle to cardiac muscle, compared to the control group, are indicated in Figure 2.
| Group | Initial Weight | Final Weight | Paired Sample T test | Independent Sample T Test |
|---|---|---|---|---|
| Placebo (n=6) | 325.87 ± 5.21 | 529.12 ± 7.32 | P = 0.008b | P = 0.014b |
| Curcumin supplement (n=6) | 325.87 ± 5.21 | 418.6 ± 6.54 | P = 0.001b | |
| Resistance training (n=6) | 325.87 ± 5.21 | 470.06 ± 6.89 | P = 0.012b | P = 0.005b |
| Resistance training + Curcumin supplement (n=6) | 325.87 ± 5.21 | 436 ± 7.12 | P = 0.000b |
Mean, Standard Deviation, Paired, and Independent T Tests of Body Weight in Groups Under Studya
As the results indicate in Table 2, curcumin supplement and resistance training increased the expression of PGC-1α compared to the control group, but the increases were not statistically significant. However, curcumin + resistance training significantly increased the expression of PGC-1α. Curcumin supplement, resistance training, and their combination decreased the expression of FOXO1, compared to the placebo group. These differences were significant only in the resistance training and curcumin supplement+resistance training groups. The curcumin supplement and resistance training groups increased the expression of MuRF-1 compared to the placebo group, but the increases were not statistically significant (P = 0.286, 0.0953). On the other hand, their combination significantly decreased the expression of MuRF-1 (P = 0.013). The three treatment groups compared to the placebo group showed decreases in the expression of Atrogin-1, although none of them were statistically significant.
| Group | PGC-1α | FOXO1 | MuRF-1 | Atrogin-1 |
|---|---|---|---|---|
| Control group | ||||
| Curcumin supplement | P = 0.553 | P = 0.114 | P = 0.953 | P = 0.213 |
| Resistance training | P = 0.191 | P = 0.010a | P = 0.286 | P = 0.490 |
| Curcumin supplement + resistance training | P = 0.001a | P = 0.001a | P = 0.001a | P = 0.075 |
| Curcumin supplement | ||||
| Resistance training | P = 0.865 | P = 0.597 | P = 0.556 | P = 0.931 |
| Curcumin supplement + resistance training | P = 0.009a | P = 0.047a | P = 0.001a | P = 0.931 |
| Resistance training | ||||
| Curcumin supplement + resistance training | P = 0.044a | P = 0.389 | P = 0.001a | P = 0.638 |
The Analysis of Variance for the Variables PGC-1α, FOXO1, MuRF-1, and Atrogin-1
The results of the ANOVA test are shown in Table 3. All the treatment groups showed decreases in the expression of CoL1 compared to the control group, which were significant in the curcumin and curcumin + resistance training groups. All the treatment groups showed significant decreases in the expression of CoL3 (P = 0.003, 0.014, and 0.019). Curcumin supplement, resistance training, and their combination significantly increased the weight ratio of the left ventricle to cardiac muscle compared to the control group (P = 0.032 and 0.041). Resistance training decreased the weight ratio of the left ventricle to cardiac muscle compared to the control group, but not statistically significantly (P = 0.083).
| Group | CoL1 | CoL3 | Cardiac Muscle Weight |
|---|---|---|---|
| Control group | |||
| Curcumin supplement | P = 0.004 | P = 0.019 | P = 0.041 |
| Resistance training | P = 0.056 | P = 0.014 | P = 0.083 |
| Curcumin supplement+ resistance training | P = 0.002 | P = 0.003a | P = 0.032 |
| Curcumin supplement | |||
| Resistance training | P = 0.031 | P = 0.163 | P = 0.073 |
| Curcumin supplement+ resistance training | P = 0.006 | P = 0.062 | P = 0.088 |
| Resistance training | |||
| Curcumin supplement+ resistance training | P = 0.001 | P = 0.037 | P = 0.236 |
The Analysis of Variance for Variables CoL1, CoL3, and Weight of the Cardiac Muscle
5. Discussion
This study showed that curcumin supplement alone or combined with eight weeks of resistance training can prevent cardiac muscle atrophy in obese rats through increasing the expression of PGC-1α, as the most important mitochondrial biogenesis molecule, and decreasing FOXO1, MAFbα, and MuRF1 as members of the ubiquitin-proteasome proteolytic pathway in cardiac muscle. In addition, the expression of Collagen type I and type III, as two important proteins in the extracellular matrix in cardiac muscle, decreased, which indicated that resistance training along with curcumin did not lead to pathologic hypertrophy and cardiac compliance.
The comparison of resistance training with 50 and 80% intensity in male athletes after the glycogen depletion of muscles showed no significant difference in the expression of PGC-1α (29). However, in 2016, Kiwata et al. reported that PGC-1α resting-state increases in healthy people after resistance training. Conversely, it did not have any effect on the expression of PGC-1α in menopausal females (30). In 2018, Menishko et al. studied the simultaneous effect of training and calorie restriction on insulin resistance and PGC-1α expression in old obese people. They showed that 16 weeks of training with medium intensity could increase PGC-1α expression (31). Our results showed that eight weeks of resistance training based on the established protocol could increase the PGC-1α expression.
Curcumin can increase mitochondrial biogenesis through gene expression in the cell, like calorie restriction through SIRT-1. Curcumin isolated from the plant Curcuma longa stimulates PGC-1α transcription by activating AMPK (26). Our results indicated that curcumin increased PGC-1α expression by 120%. The most interesting finding of the present study was the double effect of curcumin and resistance training on PGC1-α gene expression. PGC-1α and AKT signaling are the most important inhibitors of FOXO1 (8, 32, 33). The Akt/PI3K pathway is stimulated by either insulin or IGF-1 and adjusts the hypertrophy of the skeletal muscle. The activation of Akt by IGF-1 can increase the activity of the P70S6K/mTOR pathway and protein synthesis (33). Curcumin and resistance training are two factors that increase IGF-1, which enhances protein synthesis and prevents apoptosis and muscle cell atrophy through activating the PI3k/Akt pathway. Another finding of this study was a 21% decrease in FOXO1 expression in the curcumin group compared to the placebo group. Therefore, the researchers suggest that curcumin supplementation partly increases IGF-1 expression, thereby enhancing Akt activation and ultimately enhancing p70S6K/mTOR pathway activity.
As our results illustrated, the increase in the expression of PGC-1α and the decrease in the expression of FOXO1, MuRF1, and Atrogin is affected by curcumin intake and resistance training, which is in line with the earlier studies (9). Based on our results, curcumin can decrease Atrogin and MuRF1 and protect the cardiac muscle by decreasing the expression of FOXO1. These findings were in line with the results of Mukai et al. in 2010 that examined the effect of curcumin consumption on ubiquitin-induced muscle atrophy in mice.
In physiologic conditions, such as exercising, myocytes are surrounded by a delicate network of collagen. Exercise training is not accompanied by collagen pile-up in cardiac muscle (21). Collagen is one of the important proteins in the extracellular matrix framework, which determines the mechanical characteristics of the tissue. In the present study, the impact of eight-week resistance training on cardiac muscle hypertrophy and the effect of curcumin in the expression of type I and III collagens and their relations were assessed. The results indicated that type I and III collagen reduced in the three treatment groups compared to the control (placebo) group. These reductions for type I and type III collagen in the resistance training + curcumin group were 53% and 57%, respectively. They were 10% and 29% in the resistance training group and 27% and 37% in the curcumin group, respectively. Thus, resistance training combined with curcumin had the highest influence on preventing cardiac muscle hypertrophy. Moreover, both collagen I and II showed a significant decrease in three treatment groups compared to the placebo group. This shows that curcumin and resistance training could increase the dilation power of the heart muscle, improve cardiac function, and prevent cardiac atrophy.
5.1. Conclusion
According to the results, resistance training increases the expression of PGC-1α, the ubiquitin-proteasome pathway, and decreases the expression of proteolytic pathway, including FOXO1, MuRF1, and Atrogin. On the other hand, it does not lead to pathologic hypertrophy of cardiac muscle, as it reduces the expression of type I and type III collagen. Moreover, herbal compounds like phenols can prevent cardiac muscle atrophy due to their impact on signaling pathways. Curcumin is one of these compounds whose anti-inflammatory and anti-oxidant properties have been proven. As stated, curcumin, along with physical exercise can prevent cardiac muscle atrophy in obese patients, increase the expression of PGC-1α, and decrease FOXO1, MuRF1, and Atrogin. It also decreases the expression of collagens and their relations. When resistance training is accompanied by the appropriate dosage of curcumin, it can have an additional influence on preventing cardiac muscle atrophy. Needless to say, further investigations are required for determining the effective signaling pathways.