1. Background
With the highest mortality rate, breast cancer is one of the most prevalent cancers in the world, so that 502,000 women annually die due to this disease (1, 2). Based on available statistics, breast cancer accounts for approximately 33% of gynecological cancers, with an estimated prevalence of 8 to 10% in different countries. In Iran, accounting for 24.4% of all cancers, breast cancer is the first prevalent cancer in women. Recent studies in Iran have revealed that the rate of breast cancer is 17.81%, which has significantly increased in recent years (1, 3).
Breast cancer is a heterogeneous disease in clinical behavior. Pathological factors like pathology degree, tumor size, lymph node metastasis, histological type, vascular invasion, and cell proliferation rate may be effective in specifying the prognosis and the need for adjuvant therapy (4). Tumor size and lymph node status for most breast cancer patients are bio-indicators of tumor invasion and independent prognostic factors for survival after diagnosis. The tumor size and the number of positive lymph nodes in axillary autopsy are related directly. An association has been observed for decades to date in all major studies performed, with the majority of studies on the BRAC1 mutation (5, 6).
Ductal carcinoma in situ (DCIS/stage 0 breast cancer) mammographically detects about 20% of breast cancers (7). Five percent of cases in women are diagnosed before the age of 40 (8). The factors predicting mortality after diagnosis of DCIS are not known. In particular, preventing invasive recurrences using radiotherapy or extensive breast surgery (mastectomy) has not been suggested to decline the mortality rate of this type of breast cancer. Breast cancer mortality rates are low in women with DCIS, and a large group is required to be studied for a long time to accurately estimate the mortality rate.
2. Objectives
This study aimed at evaluating the association between the pathological specifications of breast cancer patients with ductal carcinoma to reduce the mortality rate in these patients
3. Methods
In this study, 50 patients with breast cancer of ductal carcinoma type were recruited. All patients were equal in terms of gender (female), not receiving chemotherapy and mastectomy. Necessary permission and ethical code were obtained from the Ethics Committee of the Islamic Azad University, North Tehran Branch, to keep the confidentiality of patient data based on the Helsinki Declaration. The patients’ data were regarded as confidential and used only for research purposes.
This was a descriptive-analytical, cross-sectional study. The statistical population included patients with a diagnosis of breast cancer referring to Rasoul Akram Hospital from June 22, 2018, to June 21, 2019. A convenience sampling was used. Data were collected by a field method and completing checklists using patients’ files. The checklist included age, sex, location of the tumor, the extent of tumor invasion to the peripheral organs, stage of involvement of lymph node, besides the metastatic involvement, tumor stage, type of tumor pathology, and tumor size.
3.1. Statistical Analysis
After recording in checklists, raw data were entered into SPSS 26 software and presented by descriptive statistics (mean, standard deviation, number, and percentage). To evaluate the differences between the size of the tumor and involvement/non-involvement of lymph nodes, the independent t test was used. To perform the t test, it was necessary to check the assumption of the homogeneity of variances in the two groups. For this purpose, Levene's test was used.
4. Results
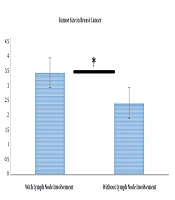
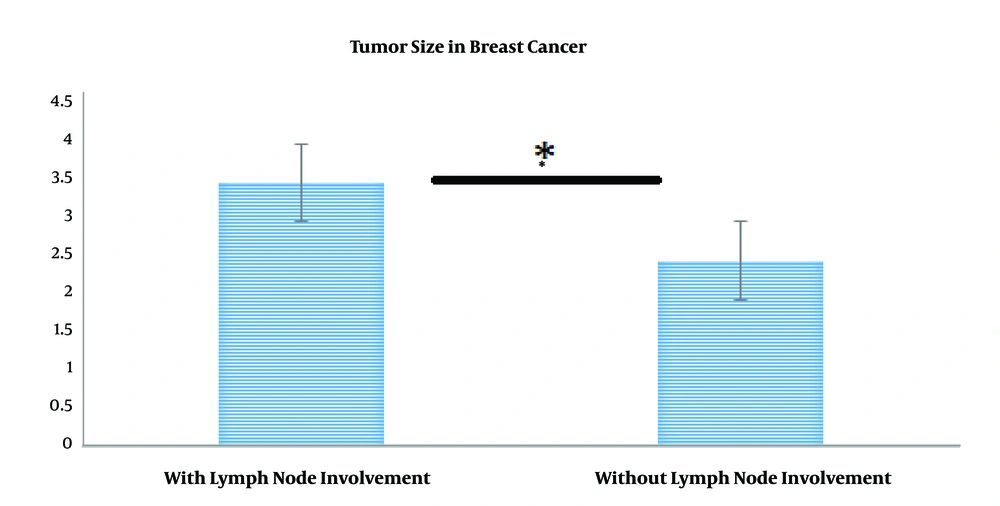
As indicated in Table 1, when lymph node involvement was present, the mean tumor size was 3.44, while it was 2.42 in the absence of involvement. To test this hypothesis, the independent t-test was used, the results of which are presented in Table 2. To assess the equality of variances, the results of Levene’s test were first assessed. As observed in Table 2, Levene’s statistic is not significant for the size variable, suggesting that the condition of the equality of variances was met. Since t = 2.72 and P < 0.05, the difference in tumor size between the two involved and non- involved lymph node groups was significant (Figure 1).
| Standard Error (SE) of the Mean | Standard Deviation (SD) | Average Tumor Size | n | Status |
|---|---|---|---|---|
| 0.42 | 1.92 | 3.44 | 21 | Lymph node involvement |
| 0.21 | 1.13 | 2.42 | 29 | Lymph node non-involvement |
| Variable | Confidence interval of 0.95 | Standard error of the mean | Compare means | Significance level | Degree of freedom (DF) | T value | Levene's test | ||
|---|---|---|---|---|---|---|---|---|---|
| Upper limit | Lower limit | Significance level | F value | ||||||
| Tumor size | 0.14 | 1.89 | 0.43 | 1.01 | 0.023 | 48 | 2.43 | 0.10 | 2.72 |
5. Discussion
Breast cancer is the most prevalent cancer among women, with an increasing rate in developing countries. In Iran, breast cancer involves women at least a decade earlier than in developed countries (9). Although specific treatments have been developed, improving the treatment results, approximately one-third of treated patients get involved with the metastatic type (10, 11). Hence, the outcome of initial treatment should be further improved, and more effective treatment strategies should be developed for recurrent and metastatic disease. The association between breast cancer and pathological factors is one of these types of strategies.
Many studies have been carried out in many countries suggesting the significance of tumor size and lymph node involvement in estimating the breast cancer prognosis (12-14). In a study conducted by Fisher et al. (1969) on 2,578 patients with breast cancer, they found an association between the size and lymph node status (5). In 1978, Valagussa et al. studied 716 patients and reported that the survival rate was directly proportional to the size of the initial tumor (15). In the same year, a linear relationship was reported by Smart et al. between the tumor size and lymph node involvement (16). An analysis of data from 24,740 breast cancer cases recorded in the SEER program of the US National Cancer Institute by Carter et al. revealed a linear relationship, as well (17). In 2017, Samavati et al. reported that among malignancies, invasive ductal carcinoma was the most prevalent malignancy in Iran (87.6% of all malignancies) with an increasing rate (18). Thus, given the increased number of patients with invasive ductal carcinoma in our country (Iran), this type of breast cancer was studied, and a direct relationship was obtained between the tumor size and lymph node status, confirming previous studies.
The age under 20 years was not observed in this study, which is in line with other studies (19-21), indicating the importance of breast masses in the ages of 40 to 60 years that are the most malignant masses in this age range (22). The mean age of 50 patients in the present study was consistent with previous studies.
5.1. Conclusion
In this study, we showed a correlation between lymph node status and tumor size in ductal breast carcinoma that may lead to metastasis and recurrence in the disease. The rate of this disease and its mortality is increasing in Iran, so it is suggested that more attention be paid to adopting preventive measures besides early diagnostic methods and assessing more risk factors in this regard.