1. Background
Nanotechnology is an interdisciplinary science that links different scientific fields, including medical and pharmaceutical technology, physics, engineering, and chemistry (1). Nanoparticles (NPs) are small-sized particles with typical dimensions of 1 - 100 nm (2, 3). Due to the small size of NPs, they can interact with a variety of biomolecules, including proteins and nucleic acids (4). These properties enable NPs to pass through the cell or not to be detected by the immune system (2). In recent years, NPs, especially metal NPs, have been widely used in biology and medicine. In biological systems, these materials are used due to characteristics such as small size, high surface area to volume ratio, high reactivity to living cells, stability at high temperatures, and effective transfer to cells (5).
There are various methods for the synthesis of NPs, the most significant of which are chemical and biological methods. The main disadvantage of these methods is the use of harsh conditions, such as temperature and pressure, and the use of highly flammable organic solvents, which are required in the production of NPs. The chemical synthesis method largely depends on the availability of metal and NP precursors (6). Nowadays, green synthesis methods have attracted lots of attention due to their biocompatibility and antioxidant properties (7) and several advantages over chemical methods (8, 9). Gold NPs are among the most widely used metal NPs, which are used in almost all fields of medicine, including diagnosis, treatment, prevention, and drug delivery (10).
Various studies have been performed on the effect of different NPs alone or in combination with some medicinal plants on the growth and development of ovarian follicles. It has been reported that the dermal contact of gold NPs (GNPs) in male mice can lead to hepatocyte nucleus deformity, cytoplasm destruction, structural changes of sinusoids, as well as Portal and Central Vienna with apoptosis and necrosis. Of course, in macroscopic discussion, but in microscopy no signs were observed according to the reference (11). The results of a study on the effects of GNPs and silver NPs (AgNPs) on the oocytes, spermatozoa, and embryos of mice showed that GNPs were cytotoxic agents but had much less toxic effects on the embryo than AgNPs (12). The results of a study in 2015 showed that the synthesis of GNPs from biogenic materials such as plant or fungal extract was highly suitable for protecting the environment and enhancing biological activities (13). However, studies have shown that the treatment of Caenorhabditis elegans with GNPs can lead to mutation in future generations; although, the exposure of GNPs to the Achillea millefolium (AM) extract can reverse these mutative effects (14).
2. Objectives
Due to the increasing use of GNPs in industry and medicine and the global problem of infertility among societies, it appears interesting to study their effects on health and, especially, on the growth and development of ovarian follicles (15, 16). The present study aimed to investigate the effects of commercially provided and green synthesized GNPs as well as the AM extract on the developmental parameters of preantral follicles (PFs) and the production of steroid hormones in NMRI mice.
3. Methods
3.1. Materials
Insulin-transferrin selenium (ITS) was obtained from Sigma-Aldrich (Poole, United Kingdom). Moreover, fetal bovine serum (FBS) and alpha MEM medium (α-MEM) were purchased from Invitrogen.
The High Pure RNA Isolation Kit and cDNA Synthesis Kit were also purchased from Roche (Mannheim, Germany) and Fermentas Inc. (Vilnius, Lithuania), respectively.
Further, the primers were obtained from Bioneer (Daejeon, Korea), and commercially provided GNPs (C-GNPs) were purchased from a nano zino company.
3.2. Collection and Identification of Plant Samples
The aerial parts of AM were collected from the northeast of Iran (the Kardeh-Dam Area, Mashhad) and identified in the herbarium of the Islamic Azad University, Mashhad Branch, Iran, with the code 10318. The aerial parts were then separated from other parts, washed twice with distilled water, and dried at room temperature for 7 days (17).
3.3. Extraction
Extraction was performed at the Applied Biology Research Center for Animal Development of the Islamic Azad University, Mashhad Branch, Iran. For this purpose, 5 grams of the powder prepared from the aerial parts of the yellow AM plants were mixed with 100 mL of sterile distilled water and then heated until boiling. After cooling, the mixture was filtered through Whatman filter paper grade 1, and the resulting extract was kept at 4°C for subsequent use.
3.4. Isolation of Ovarian Follicles
PFs were mechanically removed from NMRI mice (age 16 - 14 days) following the rules of the Bioethics Committee. For this purpose, female mice were euthanized by cervical dislocation, and then the ovaries were removed mechanically under a stereo microscope and placed in α-MEM containing 5% FBS and 1% penicillin-streptomycin. Next, the oocytes were removed from the ovarian tissue using a 26 mL needle and washed with HEPES buffer solution. Afterward, they were cultured in 1 ml of α-MEM containing 50 µL of FBS, 10 µL of ITS, and 100 µL of FSH.
3.5. Culture of PF
The isolated follicles were added to the culture medium and incubated at 37°C and 5% CO2 for 12 days. The fresh culture medium was replaced with half of the culture medium once every two days.
3.6. Follicular Groups
The follicles were treated with 10, 25, 50, and 100 µg/mL of C-GNPs, green GNPs, and the AM extract. After the cytotoxicity evaluation, 50 µg/mL of each study groups was used for further examination.
1) The control group did not receive any treatment.
2) In the experimental group 1, only the AM extract was added to the samples.
3) The experimental group 2 was treated with green GNPs.
4) The experimental group 3 was treated with C-GNPs.
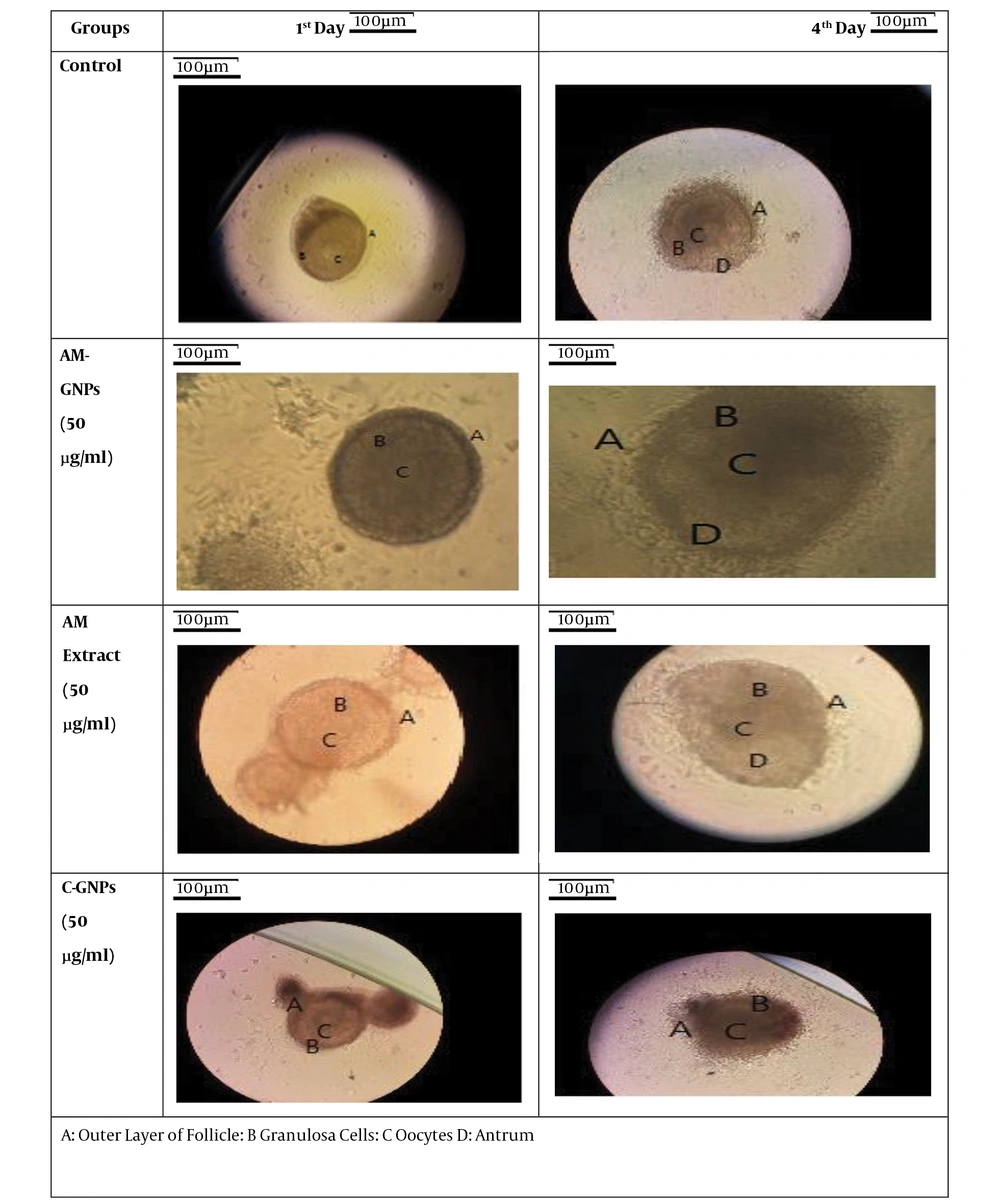
The follicles were photographed using an inverted microscope equipped with a camera (Biomed Korea) in the first four days of culture.
3.7. Histological Studies
3.7.1. Sampling and Tissue Preparation
In a sterile environment, the mice’s bodies were cleaned with 70% alcohol, and after making an incision in their bodies, the ovaries were first placed in physiological saline and then under a microscope. Afterward, the ovaries were separated from the surrounding tissues and used for histotechnical procedures. The ovaries were fixed in standard fixation solutions (formalin 10%) after washing. Next, the dehydration step was performed using different dilutions of ethanol (70%, 80%, 90%, 96%, and absolute ethanol) for 90 min for each dilution (18, 19).
3.8. Measurement of Hormone-related to Mouse Ovarian Function
The estradiol level was evaluated in the experimental groups (dose of 50). For this purpose, after blood sampling from the heart, the amounts of the hormone were measured using radioimmunoassay and compared to that in the control group. To measure the estradiol hormone, 20 follicles in the control and experimental groups were treated with 50 µg/mL of the AM extract, green GNPs, and C-GNPs. By sampling from the culture medium, the estradiol amount was determined separately in each group with the radioimmunoassay method using ELISA (Bio rad, US).
3.9. Evaluation of Morphometric
After the treatment, the samples were incubated at 37°C and 5% CO2 for 12 days. The photographs were taken 4 days after the treatment. During follicle culture, variables such as the growth rate of granulosa cells and the antral cavity formation were evaluated every two days using an inverted microscope (Biomed Korea).
3.10. Statistical Analysis
The statistical analysis was performed using the SPSS 16.0 statistical package (SPSS, IL, US). A P-value < 0.05 was considered significant. The data were analyzed using ANOVA followed by the Tukey’s test.
4. Results
4.1. Morphometric Indices
The FTIR analysis showed that the green NPs were synthesized using the plant extract, and that the size of GNPs was 20 nm. In this study, the effects of the AM extract, AM-GNPs, and C-GNPs were investigated on the PF growth parameter. The results showed that in the control group, PF was surrounded by a partial or complete layer of squamous granulosa cells on the first day of the treatment. The morphology of the follicles during the culture period showed that the follicles were attached to the bottom of the culture flask and were immobile. Moreover, on the fourth day, the granulosa cells spread around the follicles, forming an irregular shape of follicles (Figure 1).
PF treated with the AM extract had the same characteristics as the control group on the first day. The follicles were treated with the aqueous AM extract at concentrations of 10, 25, 50, and 100 µg/mL; however, follicles at concentrations above 100 µg/mL of the AM extract were not attached to the bottom of the flask and were degenerated. The best outcome was found at 50 µg/mL, in which the growth of the granulosa cells was greater than the control group on the second day. Further, on the fourth day, the number of granulosa cells increased significantly compared to the control group. These follicles had one or two small areas of follicular fluid accumulation. On the sixth day, due to the increasing growth of the granulosa cells, the size and further details of the follicles were not visible.
PF treated with green GNPs contained an oocyte on the first day and was surrounded by a thin layer of granulosa cells. The follicles were cultured at 10, 25, 50, and 100 µg/mL of AM-GNPs. On the second day, the granulosa cells grew slightly at all the concentrations. On the fourth day, the granulosa cell layers were almost identical to the control with no significance at 10 µg/mL. but at concentrations above 50 µg/mL, the follicles degenerated and detached from the bottom of the flask.
PF treated with C-GNPs (10, 25, 50, and 100 µg/mL) contained an oocyte on the first day and was surrounded by the granulosa cells. On the second day, the granulosa cells grew slightly at all the concentrations. The granulosa cell layers grew less compared to the control group, and most of the follicles degenerated in the following days of culture. Moreover, antrum was not formed at any concentration of C-GNPs.
4.2. Mean Changes in Follicle Diameter
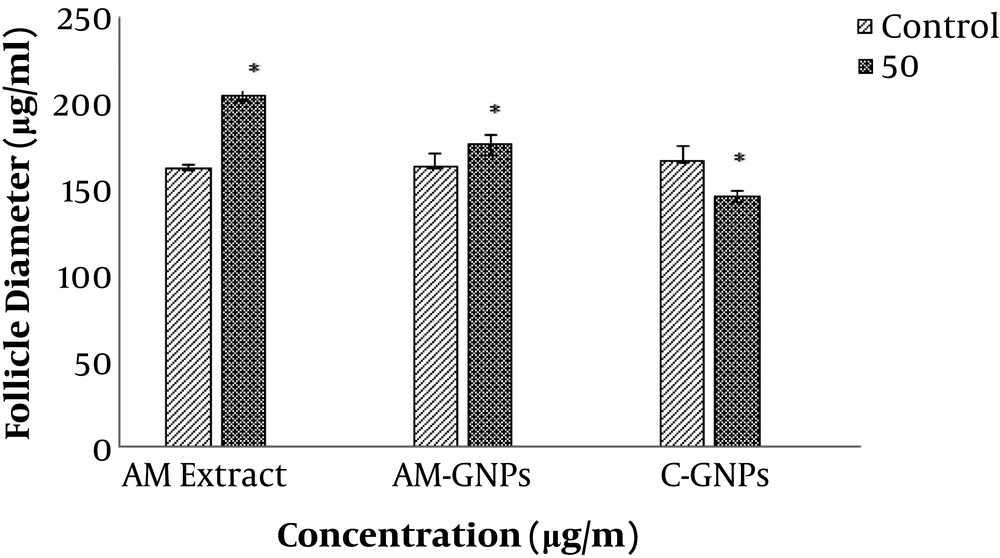
The evaluation of the mean diameter of PF treated with the aqueous AM extract showed that the mean diameter of the follicles at the 50 μg/ml concentration of the AM extract increased significantly in days one to four compared to the control group (P < 0.05). The evaluation of the mean diameter of the follicles treated with green GNPs showed that the mean diameter of the follicles significantly increased at the 50 µg/mL concentration of the NPs on days 1 to 4 compared to the control group. The mean increase in the follicle diameter in this group was lower compared to that in the AM extract-treated group (P < 0.05), as shown in Table 1.
| Groups | Day 1 µg/mL | Day 5 µg/mL |
|---|---|---|
| Control | 600 ± 0.00 | 24100 ± 0.00 |
| AM extract, 25 µg/mL | 710.5 ± 1.61 | 20550.17 ± 0.96 |
| AM extract, 50 µg/mL | 810.13 ± 1.20 | 25920.1 ± 0.49 |
| AM-GNPs, 25 µg/mL | 670.1 ± 1.25 | 12470.07 ± 0.70 |
| AM-GNPs, 50 µg/mL | 700.17 ± 1.85 | 17850.17 ± 1.16 |
| C-GNPs, 25 µg/mL | 590.03 ± 2.15 | 13810.1 ± 0.95 |
| C-GNPs, 50 µg/mL | 530.43 ± 2.87 | 12005.3 ± 1.67 |
aValues are expressed as mean ± SD.
The evaluation of the mean diameter of PF treated with C-GNPs one to four days after the treatment showed that the mean diameter of follicles treated with the 50 µg/mL concentration of C-GNPs was significantly reduced compared to the control group. The decrease in the follicle diameter was due to the atresia of the treated follicles, which raised with increasing the concentration (P < 0.05).
5. Discussion
In the present study, the effects of GNPs, provided through the commercial market using chemical and green synthetic methods, and the aqueous AM extract were evaluated on the PF growth. The follicles consisted of oocytes and a layer of squamous follicular cells. However, the follicles remained in the meiotic division stage until fertilization. In the late stage of the growth of primary follicles, zona granulosa was formed by proliferating follicular cells between the oocyte and the follicular cells. When secondary follicles were formed, the follicular antrum appeared in the granulosa cells, the number of granulosa cell layers increased, and large oocytes were observed. The oocyte was located in the center of the secondary follicles after the first meiosis (20). The above-described developmental stages were to prepare PF for fertilization, which can also be performed in the extracellular environment. The effect of different materials on follicular development can be studied using IVM. Materials with a positive effect on follicular development can enhance oocyte maturation and shorten the maturation period, while harmful substances can cause atresia or delay the growth and development of follicles. Therefore, in the present study, the growth and development of PF treated with AM-GNPs, C-GNPs, and the AM extract were evaluated for morphological changes and the follicle diameter. The results showed that the plant extract at 50 µg/mL improved the PF growth and development. Previous studies have also shown that plant extracts at appropriate concentrations can improve PF growth and development. In a similar study, the effects of the Papaver rhoeas L. root extract were investigated on the PF growth and development. The results showed that the plant extract increased the PF maturation in mice, increased the diameter of follicles, increased antrum formation, and improved developmental parameters in PF compared to control follicles (21). Moreover, the effects of the aqueous saffron extract were investigated on the PF maturation. The results revealed that follicles treated with this extract had more effective growth and development than the untreated samples. Moreover, the percentage of successful fertilization was higher in the treated follicles than in the controls (22).
The morphometric and developmental evaluation of PF treated with AM-GNPs, and C-GNPs demonstrated that AM-GNPs at 50 µg/mL increased the PF diameter and had positive effects on the development of follicles. The findings showed that C-GNPs had higher toxicity than AM-GNPs on PF. In a study, the effects of GNPs with safe coatings and two different sizes (6 nm vs. 20 nm) at two different concentrations (10 µg/mL vs. 30 µg/mL) were investigated on PF maturation. The results showed that none of these GNPs had any effect on oocyte maturation and granulosa cell proliferation (12). In another study, it was shown that GNPs with a 10 µg/ml concentration did not have any significant effects on the PF development (23). According to previous studies, the toxicity of NPs, including GNPs depends to a large extent on the type of surface coating, and the size of NPs and the use of biocompatible materials can reduce the toxicity of NPs (24). In the present study, according to the FTIR spectroscopy results, a layer of plant compounds covered the surface of NPs to moderate their toxic effects. Accordingly, GNPs with the green synthesis method at 50 µg/ml (much higher concentration than other reports) were non-toxic and improved the PF developmental parameters. While in C-GNPs, the concentration of 50 µg/mL caused atresia and reduced the diameter of follicles. Moreover, the percentage of free radicals was investigated in samples treated with the AM extract, C-GNPs, and AM-GNPs. The results showed that the percentage of free radical in samples treated with the AM extract and AM-GNPs was closer to the control level. However, the percentage of free radicals increased in follicles treated with C-GNPs, indicating that GNPs covered with the plant extract could, to some extent, inhibit free radicals. This beneficial effect of green GNPs can be attributed to the AM extract, which has significant antioxidant potential (25). However, in C-GNPs, the percentage of free radicals increased due to the lack of antioxidant coating.
Another indicator of the PF development is the secretion of related hormones, such as estradiol and progesterone. Estradiol is synthesized in granulosa cells and is essential for the development and fertilization of follicles (26). This study results demonstrated that the amount of this hormone increased in samples treated with the AM extract and AM-GNPs compared to the control samples but decreased in follicles treated with C-GNPs. On the other hand, the mean of progesterone showed a significant reduction in all the experimental groups compared to the control group (Figure 2).
In a recent study on the effect of other NPs such as AgNPs on Sertoli and granulosa cells in male and female mice, the formation of autophagosome and autolysosome was observed in Sertoli cells. Apoptosis was also observed due to the release of cytochrome C in the mitochondria of cells treated with AgNPs and inflammatory cytokines such as tumor necrosis factor A and interferon 6 significantly increased, leading to the destruction of male and female gametes (27). In a similar study, GNPs were synthesized using the Juglans regia extract as a natural antioxidant and stabilizing agent. The results of cytotoxicity evaluation showed that these NPs with dose-dependent toxicity could be suitable candidates for various medical applications and pharmaceutical purposes (28). One study also investigated the anti-cancer activity of GNPs synthesized using the Thyme (Thymus Vulgaris) leaf extract against HeLa cells. The results showed that GNPs synthesized with this extract inhibited the proliferation of HeLa cells in a dose-dependent manner and also induced cell death through the caspase-dependent pathway (29).
A study in 2013 showed that AM had a variety of healing properties. The results of a study on the anti-cancer properties of this plant in the K562 cell line showed that the AM extract caused the death of K562 cells dose- and time-dependently. Accordingly, the extract caused morphological differences in the cells at lower concentrations, whereas it led to cell cycle arrest and plasma membrane disintegration at higher concentrations. Therefore, experimental findings indicated the therapeutic potential of the AM extract, which is mediated by inducing differentiation as well as inhibiting cell cycle and apoptosis in cancer cells (30).
5.1. Conclusions
In this study, estradiol levels increased in all the experimental groups in different GNP concentrations. However, these NPs led to a decrease in progesterone levels in all the groups at similar concentrations. Moreover, unlike green gold nanoparticles, commercial nanoparticles have been reduced in size by 50 doses.