1. Background
Plasmodium species causes a parasitic disease named malaria. Malaria in humans is caused by Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, Plasmodium malariae, and Plasmodium knowlesi. Plasmodium falciparum is the most common and the primary cause of malaria incidence clinically. According to the 2019 malaria report, there were 228 million new cases and 405,000 deaths in 2018 worldwide (1). Malaria is common in tropical and sub-tropical regions around the world. However, some population groups (children < 5 years, pregnant women, patients with sickle cell and HIV/AIDS, and non-immune immigrants) are significantly at a higher risk of contracting the infection and developing severe complications (2). Malaria is prevalent in Nigeria, accounting for approximately 60% of outpatient visits to medical facilities, 30% of infant mortality, and 11% of maternal deaths (3).
The use of traditional medicinal plants has played a significant role in the treatment of malaria for thousands of years in different parts of the world. The most potent antimalarial drugs, quinine, and artemisinin, were isolated from Cinchona (Rubiaceae) and Qinghaosu (Asteraceae), respectively (4). The emergence of resistance to various antimalarial drugs such as quinolones, antifolates, and artemisinin derivatives has been reported (5), leading to a major setback in the management of malaria infection.
Medicinal plants are believed to be safe, with no toxic effects (6). However, some plants are naturally toxic, leading to adverse reactions. Most medicinal plant products used in folk remedies have scientific-based evidence on their pharmacological activities, with little or no information on their safety profile (7). Therefore, toxicity studies should be carried out on all medicinal plants to determine and establish their short and long-term safety.
Detarium microcarpum grows naturally in the drier regions of West and Central Africa. It is known locally as taura (Hausa), ofo (Igbo), ogbogbo (Yoruba), gatapo (Kanuri), gkungorochi (Nupe), aikperlarimi (Etsako), and gwogwori (Gwari). It is used traditionally for the treatment of malaria, rheumatism, urogenital infections, hypertension, stomachache, leprosy, and impotence (8). In Nigeria, D. microcarpum is used for the treatment of malaria by the Lala tribe (9) and the Gwaris (10), but its efficacy has not been scientifically investigated. Previous scientific work on D. microcarpum revealed that the plant possesses antimicrobial activity (11), anti-inflammatory and analgesic effects (12), as well as anti-bacterial activity (13).
Chloroquine sensitive Plasmodium berghei berghei, a rodent malaria parasite model, is used to evaluate the in vivo antimalarial activity (14). Most conventional antimalarial drugs, such as chloroquine, halofantrine, mefloquine, and artemisinin derivatives, have been developed using this model (15).
2. Objectives
The purpose of this research was to evaluate the antiplasmodial potential and safety profile of the methanol extract of D. microcarpum stem bark.
3. Methods
3.1. Plant Material Collection and Authentication
Detarium microcarpum fresh stem barks were collected from Gwarzo village, Gwarzo LGA, Kano, Nigeria, and identified and authenticated at the herbarium unit of Bayero University Kano, Nigeria. A voucher specimen number 0071 was collected for future reference and compared with the already deposited plant specimen.
3.2. Preparation of the Extract
Stem barks were air-dried under shade at room temperature until the attainment of constant weight. Then, 1000 g of the powdered material was extracted in four liters of 70% v/v methanol for 72 hours using the cold maceration process with regular shaking. The liquid methanol extract was filtered and evaporated in an oven at a temperature of about 45°C.
3.3. Experimental Animals
Adult Swiss albino mice (16 - 22 g) and Wistar rats (170 - 200 g) of both sexes were procured from the Department of Pharmacology and Therapeutics animal house facility, Bayero University Kano. They were kept for a week to acclimatize and maintained in standard laboratory conditions in conformity with the National Academy of Sciences' guidelines for the care and use of laboratory animals (1996).
3.4. Drugs and Chemicals
Chloroquine (Fluka, Germany), artesunate (Mekophar, Vietnam), pyrimethamine (SKG, Nigeria), methanol (JHD Sci-Tech. Co. Ltd, China), Agappe Diagnostic kit (Switzerland), and Randox Diagnostic kit (UK) were used in this study.
3.5. Plasmodium Parasite
Plasmodium berghei berghei, which is chloroquine-sensitive, was obtained from the National Institute of Medical Research (NIMR) in Yaba, Lagos, Nigeria. It was preserved by continuous intraperitoneal inoculation from a donor mouse to a fresh mouse after every four days (16, 17).
3.6. Parasite Inoculation
A P. berghei berghei-infected mouse with a parasitemia level of 34% was used as a parasite donor. The blood sample was collected retro-orbitally into an ethylenediaminetetraacetic acid (EDTA)-containing bottle, and the percentage of parasitemia and red blood cell (RBC) count were determined. The inoculum was prepared by diluting the blood with isotonic saline (18) to be administered intraperitoneally; as a result, approximately 1 × 107 parasitized RBCs were present in 0.2 mL of the blood solution.
3.7. Preliminary Phytochemical Screening
Using established methods, the preliminary phytochemical analysis of the extract was conducted to determine the presence of secondary metabolites (19).
3.7.1. Acute Toxicity Study (LD50 Determination)
Lorke's method was used to determine the acute oral toxicity of the extract (20). The study was conducted in two phases. In the first phase, three groups of three mice were given the extract doses of 10, 100, and 1000 mg/kg and were observed for the first four hours, and then after 24 hours for signs and symptoms of toxicity, including death. In the second phase, three groups of one mouse each were given 1600, 2900, and 5000 mg/kg of the extract and were observed for the first four hours, and then after 24 hours for signs and symptoms of toxicity, including death. The geometric mean of the lowest lethal dose (1/1) and the highest non-lethal dose (0/1) was used to calculate the LD50.
3.7.2. Curative Test
The activity of the extract against established infection was assessed using the method described by Ryley and Peters (21). On the first day (D0), mice were inoculated with P. berghei berghei. They were randomly divided into six groups (n = 6) 72 hours later (D3). Group I received 10 mL/kg of distilled water (negative control), Group II received 200 mg/kg of the extract, Group III received 400 mg/kg of the extract, Group IV received 800 mg/kg of the extract, Group V received 10 mg/kg of chloroquine, and Group VI received 5 mg/kg of artesunate (positive control) orally for five days (D3–D7). The blood was collected from each mouse by tail-bleeding on the third (D3–post-parasite inoculation) and seventh (D7–post-treatment) days. The microscopic examination of a Giemsa-stained thin blood smear was used to determine parasitemia. Over a 28-day period (D0–D27), the average survival time (days) of the mice in each treatment group was determined.
3.7.3. Peters 4-Day Suppressive Test
The extract was screened using the method described by Peters (22) against early infection. On the first day (D0), mice were divided into six groups (n = 6) at random and were infected with the parasite. Four hours after inoculation, Group I received 10 mL/kg of distilled water (negative control), Group II received 200 mg/kg of the extract, Group III received 400 mg/kg of the extract, Group IV received 800 mg/kg of the extract, Group V received 10 mg/kg of chloroquine, and Group VI received 5 mg/kg of artesunate (positive controls) orally. The animals were treated for four days (D0–D3). Blood was collected from each mouse by tail-bleeding on the fifth day (D4), and the microscopic examination of Giemsa-stained thin blood smear was used to determine parasitemia.
3.7.4. Prophylactic (Repository) Test
The method described by Peters (22) was used to evaluate the activity of the extract. Mice were divided into five groups (n = 6) randomly. Group I received 10 mL/kg of distilled water (negative control), Group II received 200 mg/kg of the extract, Group III received 400 mg/kg of the extract, Group IV received 800 mg/kg of the extract, and Group V received 1.2 mg/kg of pyrimethamine (positive control) orally for five days (D0–D4). The mice were inoculated with the parasite on the sixth day (D5), and 72 hours later, the blood was collected by tail-bleeding. The microscopic examination of Giemsa-stained thin blood smear was used to determine parasitemia.
3.7.5. Parasitemia Determination
Thin blood smears were placed on microscope slides, fixed for 10 minutes with absolute methanol, and stained for 30 minutes with 10% Giemsa stain. The slides were gently washed and dried at room temperature. A light microscope with an oil immersion eyepiece of 100x magnification power was used to count the number of parasitized erythrocytes. Each slide had three fields counted, and the average was calculated (23). The average percentage of parasitemia and the percentage of suppression were calculated as follows:
where Po is the average parasitemia in the control group, and Pt is the average parasitemia in the treated group.
3.8. Sub-chronic Toxicity Study
The procedure followed the OECD 407 guidelines (24). Rats were divided into four groups (n = 6) at random. Group I received distilled water (control), Group II received 200 mg/kg, Group III received 400 mg/kg, and Group IV received 800 mg/kg of the extract orally for 28 days. They were visually observed for changes in behavioral patterns, physical appearance, symptoms of illness, and mortality. The animals were euthanized, and a blood sample was collected for the determination of biochemical and hematological parameters.
3.8.1. Biochemical Analysis
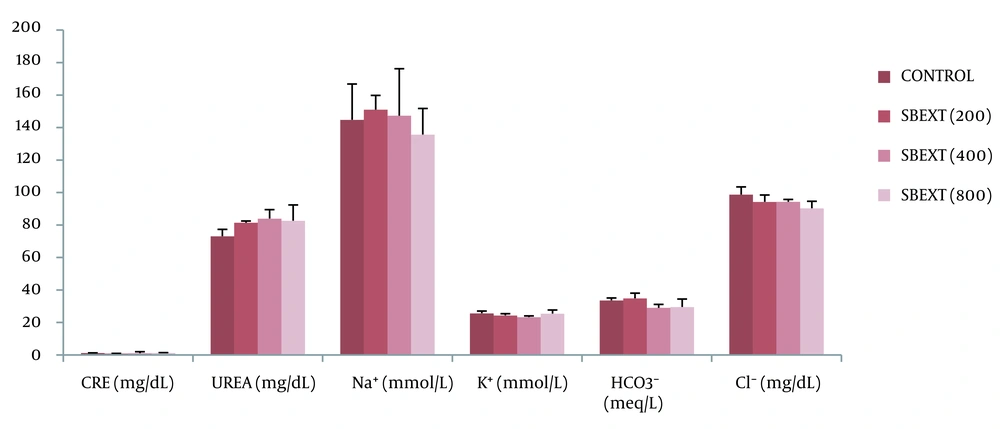
To obtain the serum, a blood sample was taken in plain dry bottles, allowed to clot, and then centrifuged at 3500 rpm for 10 minutes. The Reitman and Frankel method was used to measure alanine amino transferase (ALT) and aspartate amino transferase (AST) (25). The Rec method was used to determine alkaline phosphatase (ALP) (26). Total protein (TP) was assayed by the biuret method of Gornall et al. (27). Albumin (ALB) was determined by the method of Doumas et al. (28). Bilirubin was assayed by the method of Jendrassik and Grof (29). Urea was determined by the method of Kaplan (30), and creatinine was determined by the method of Butler (31). Sodium (Na⁺) was determined by the method of Trinder (32). Potassium (K⁺) was determined by the method of Henry et al. (33). Chloride (Clˉ) was determined by the method of Schonfeld and Lewellen (34), and the method of Forrester et al. (35) was used to measure bicarbonate (HCO3⁻).
3.8.2. Hematological Analysis
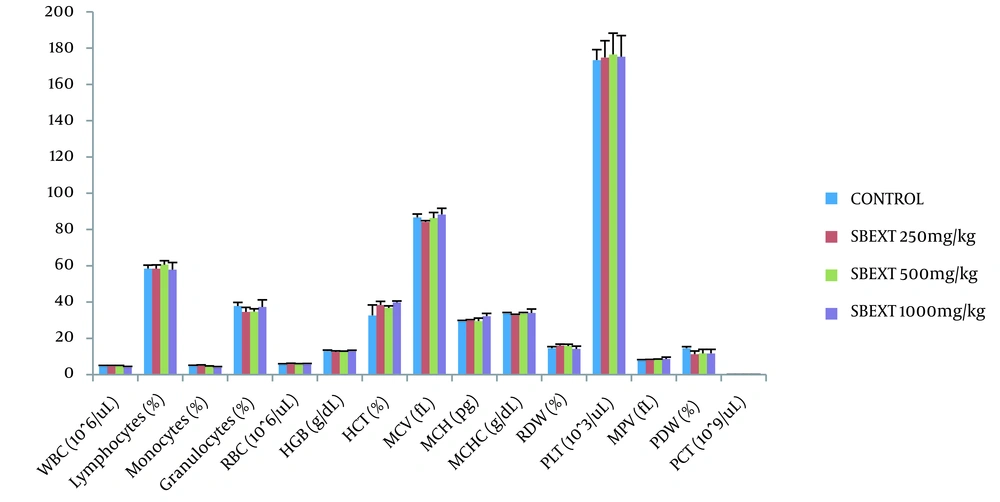
To prevent clotting, the blood was collected in EDTA bottles. White blood cell (WBC), lymphocytes, monocytes, granulocytes, red blood cell (RBC), hemoglobin (HGB), hematocrit (HCT), mean cell volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), platelet (PLT), mean platelet volume (MPV), platelet distribution width (PDW), and plateletcrit (PCT) were analyzed using a Biobase automatic hematological analyzer (BK6300).
3.9. Data Analysis
Data were expressed as the mean ± standard error of mean (S.E.M). Analysis was done using ANOVA, followed by post hoc tests (Dunnett’s test), with SPSS Statistics for Windows, version x.0 (SPSS Inc., Chicago, III., USA)”. The P values ≤ 0.05 were considered to be statistically significant.
4. Results
4.1. Phytochemical Screening
Alkaloids, glycosides, flavonoids, saponins, triterpenes, and tannins were found in the extract (Table 1).
| Constituents | Inference a |
|---|---|
| Alkaloids | + |
| Glycosides | + |
| Flavonoids | + |
| Saponins | + |
| Triterpenes | + |
| Tannins | + |
a+: Present
4.2. Acute Toxicity Study
The oral LD50 of the extract was estimated to be 3,800 mg/kg.
4.3. Curative Test
The extract at the doses of 200, 400, and 800 mg/kg produced 83.34%, 90.03%, and 86.43% parasite clearance, respectively. When compared to the negative control group, the extract showed a significant (P < 0.001) decrease in the average percentage of parasitemia level in the treated groups. Chloroquine (10 mg/kg) and artesunate (5 mg/kg) also showed a significant (P < 0.001) decrease in the average percentage of parasitemia level. The extract significantly (P < 0.001) prolonged the survival time of mice in each treated group (Table 2).
| Treatment (mg/kg) | Average Percentage of Parasitemia | Clearance Percentage | Mean Survival Time (Days) | |
|---|---|---|---|---|
| Pre-(D3) | Post- (D7) | |||
| D/W 10 mL/kg | 42.74 ± 2.45 | 84.96 ± 3.35 | - | 5.50 ± 0.30 |
| SBEXT (200) | 44.11 ± 0.85 | 15.00 ± 2.13 a | 82.34 | 16.00 ± 0.40 a |
| SBEXT (400) | 43.61 ± 0.64 | 8.24 ± 0.48 a | 90.30 | 22.33 ± 0.50 a |
| SBEXT (800) | 42.89 ± 0.70 | 11.53 ± 0.79 a | 86.43 | 18.17 ± 0.30 a |
| CQ (10) | 44.45 ± 0.92 | 9.47 ± 0.85 a | 88.85 | 27.00 ± 0.30 a |
| ART (5) | 43.62 ± 0.68 | 7.59 ± 0.52 a | 91.07 | 27.33 ± 0.30 a |
Abbreviations: D/W, distilled water; SBEXT, stem bark extract; CQ, chloroquine; ART, artesunate, D3, day 3; D7, day 7.
aP < 0.001, n = 6.
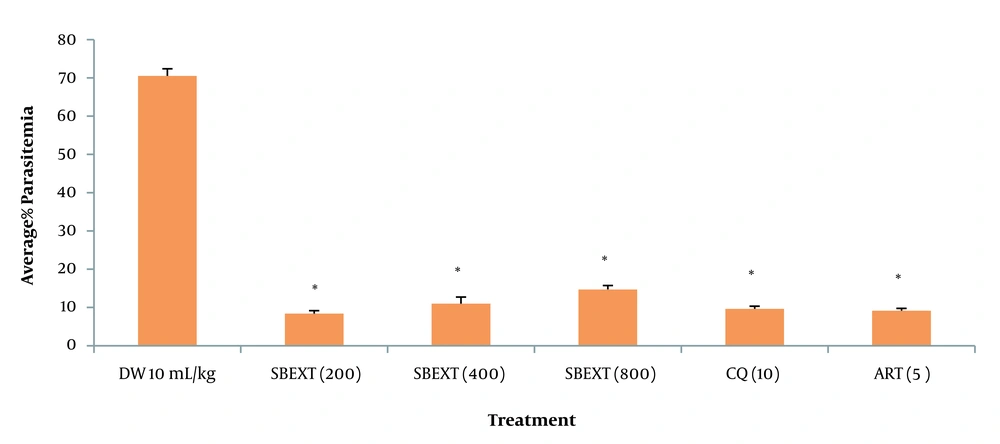
4.4. Suppressive Test
At the doses of 200, 400, and 800 mg/kg, the extract showed a significant (P <0.001) reduction in the average percentage of parasitemia level, with the highest percentage of parasite clearance observed at 200 mg/kg when compared to the negative control group. Chloroquine and artesunate also generated a significant (P < 0.001) decrease in the average percentage of parasitemia level (Figure 1, Appendix 1).
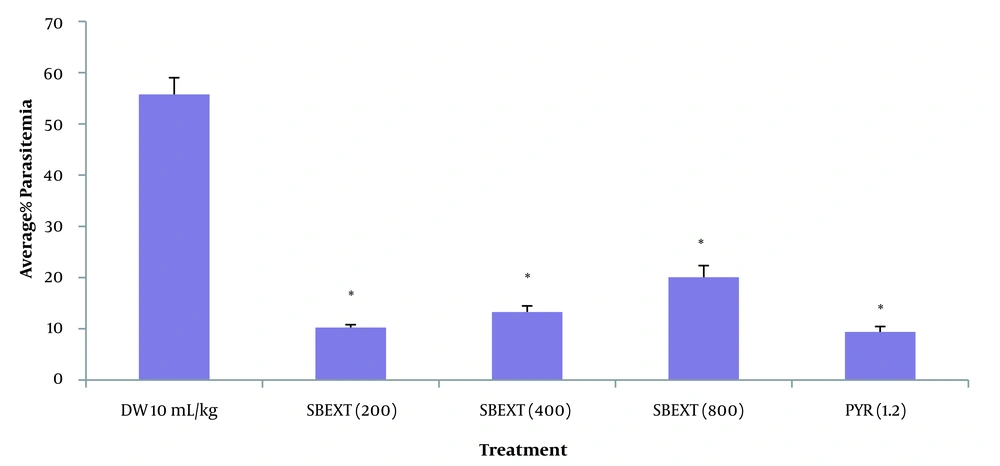
4.5. Prophylactic Test
The extract showed a significant (P > 0.001) decrease in the average percentage of parasitemia level at the doses tested (200, 400, and 800 mg/kg) when compared to the negative control group, with the highest percentage of parasite clearance observed at 200 mg/kg. Pyrimethamine produced a significant (P > 0.001) decrease in the average percentage of parasitemia (Figure 2, Appendix 2).
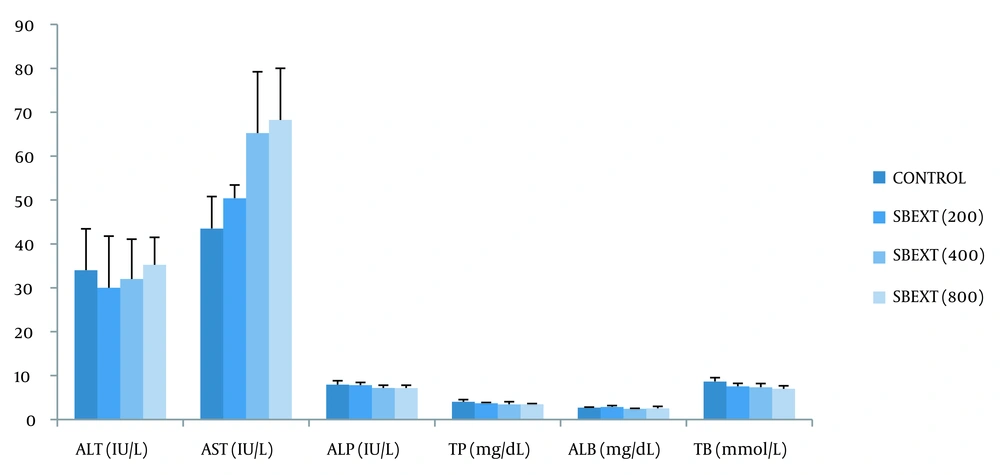
4.6. Liver Function Test
When compared to the control group, the extract produced an insignificant (P > 0.05) change in the level of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total protein (TP), albumin (ALB), and total bilirubin (TB) in the treated groups (Figure 3, Appendix 3).
4.7. Kidney Function Test
There was an insignificant (P > 0.05) change in the serum level of urea, creatinine, and electrolytes (sodium, potassium, chloride, and bicarbonate) in the treated groups when compared to the control group after the administration of the extract (Figure 4, Appendix 4).
4.8. Hematological Analysis
The extract did not significantly (P > 0.05) cause an alteration in the analyzed hematological parameters in the treated groups when compared to the control group (Figure 5, Appendix 5).
Effect of the extract on hematological parameters after 28 days of oral administration. WBC, white blood cell; RBC, red blood Cell; HGB, hemoglobin; HCT, Hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red cell distribution width; PLT, platelet; MPV, mean platelet volume; PDW, platelet distribution width; PCT, plateletcrit; SBEXT, stem bark extract; n = 6.
5. Discussion
The phytochemical analysis of D. microcarpum reported in earlier studies revealed the presence of saponins, carbohydrates, tannins, alkaloids, glycosides, cardiac glycosides, flavonoids, and triterpenes (11, 13, 36, 37). In this study, alkaloids, saponins, tannins, flavonoids, glycosides, and triterpenes were found in the methanol stem bark extract of D. microcarpum, which have numerous pharmacological activities. An acute toxicity study is conducted to determine the dose that causes mortality or serious toxicity in 50% of the animals within a specific time so as to establish the dose to be used in the subsequent study (12). The oral LD50 of the extract was determined to be 3800 mg/kg. According to (20) toxicity scale, the extract is practically safe.
The activity of the extract against established and early infection was evaluated using the curative test and the Peters 4-day suppressive test, respectively. Chloroquine, quinine, and artemisinin derivatives are some drugs used in the treatment of malaria. Chloroquine inhibits the formation of hemozoin, thereby preventing its polymerization (38). Artemisinin derivatives cause the alkylation of the heme and proteins in the parasite (39), and inhibit sarcoplasmic reticulum Ca2+ transporting ATPase (SERCA) of the parasite (40). The extract showed significant blood schizonticidal (curative) and tissue schizonticidal (suppressive) effects on the parasite density at the doses tested. The prolongation of MST of the infected mice by the extract indicates its ability to significantly suppress the infection caused by the parasite. In the antimalarial study, any test compound that can extend the MST above 12 days is considered as having a good suppressive activity (15, 41). Pyrimethamine is an antimalarial drug used in the treatment and prevention of malaria infection. It inhibits the enzyme dihydrofolate reductase (DHFR) in the parasite, preventing the reduction of dihydrofolate to tetrahydrofolate that is needed for the synthesis of amino acids and nucleic acids (42). The extract showed a significant prophylactic effect comparable to that of pyrimethamine; thus, the extract may contain agents that have prophylactic activity. The antiplasmodial effect of D. microcarpum may be due to the presence of constituents such as alkaloids, saponins, flavonoids, triterpenes, and tannins, which have been reported to be responsible for antimalarial activity in some plants and may exert their various functions through one or more mechanisms (43-46).
It is important to know the condition of the liver and the kidney when determining the safety or toxicity of a new compound. The liver is a vital organ of drug metabolism and elimination, and its normal function can be evaluated by determining the activities of various serum markers, including ALT, AST, ALP, total protein, albumin, and bilirubin. As known, ALT is a liver cytoplasmic enzyme (47), and AST is a cytoplasmic and mitochondrial enzyme found in various tissues (liver, kidney, heart, skeletal muscles, and brain) (48). Increased levels of ALT and AST indicate hepatocellular damage. Besides, ALP is a hydrolase enzyme found in cells that line the liver's biliary ducts, kidneys, bone, placenta, and intestine (49). Increased levels of ALP indicate hepatobiliary damage and liver cholestasis (50). Bilirubin is a waste product of hemoglobin breakdown, and its increased level is related to biliary cirrhosis and hepatic cholestasis (51). Serum ALT, AST, ALP, TP, ALB, and TB levels in the treated groups were insignificantly different from those in the control group. The kidney is a major organ of waste products excretion. Kidney damage can be determined by evaluating the level of serum urea, creatinine, and electrolytes. Urea is a waste product of protein breakdown, and its increased level indicates a harmful effect on renal tubules, renal parenchyma, dehydration, shock, and blockage of the urinary outflow (52). Creatinine is a waste product of muscle breakdown, and its increase is related to impaired glomerular filtration (52). When compared to the control group, an insignificant change in the level of urea, creatinine, and electrolytes in the treated groups was observed. This could imply that the extract had no toxic effect at the doses tested and was considered safe for the liver and kidneys.
The hematopoietic system is a very susceptible target for toxic substances (53). Hematological parameters can be used to determine the harmful effects of foreign compounds, such as plant extracts, on blood constituents (54). When the treated groups were compared to the control group, there was no significant change in the analyzed hematological parameters. The observed differences have no toxicological value and may signify that the extract at the doses tested is safe.
5.1. Conclusions
The methanol stem bark extract of D. microcarpum possesses significant antiplasmodial activity, thus supporting the ethnomedicinal use of the plant in the treatment of malaria infection. The assessment of biochemical and hematological parameters suggests that the extract is relatively safe at the doses tested after short-term exposure.