1. Background
Polycystic ovary syndrome (PCOS) is a multidimensional hormonal disorder representing heterogeneous clinical signs. Some of the symptoms include increased androgen production (hyperandrogenism), menstrual disorders, infertility, chronic ovulation, cystic ovaries, menstrual irregularities, a primary defect in the hypothalamic-pituitary axis (abnormal gonadotropin secretions), insulin resistance, and hair loss (1-3). The prevalence of PCOS is about 5 - 10% in different populations (4). Hyperandrogenemia, hyperinsulinemia, insulin resistance, and chronic anovulation are the major heterogeneous characteristics comprising a complex syndrome. Impaired ovarian function in patients with PCOS may cause menstrual disorder as the most common sign (5).
Hirsutism, acne, hair loss, and infertility can all be found in women with PCOS as the main symptoms of hyperandrogenism. Some possible severe complications in these patients include endometrial and breast cancers, dyslipidemia, hypertension, cardiovascular disease, and diabetes. The prevalence of obesity is about 40% in this group of women. Early diagnosis and treatment can prevent long-term complications, such as type 2 diabetes, cardiovascular disease, and myocardial infarction. However, the main reason for PCOS is unknown yet (6).
PCOS characteristics entail polycystic ovaries diagnosed on ultrasound and the clinical and biochemical symptoms of hyperandrogenism (7). Adams et al. stated that PCOS has key diagnostic criteria revealed by transvaginal ultrasound that appears more than ten cysts 2 - 8 mm in diameter arranged around an echo-dense stroma (8). Takahashi et al. mentioned that ovarian volume higher than 6.2 mm and follicles number equal to or more than 10 (in 2 - 8 mm diameter) are ultrasonic criteria for PCOS diagnosis observed in 94% of PCOS cases (9).
It is pertinent to mention that some other problems, such as the endocrine syndrome of hyperandrogenism and anovulation, may cause the same ovarian morphology and should be ruled out (10). Therefore, PCOS is diagnosed based on the clinical and/or biochemical evidence of hyperandrogenism, along with ruling out other reasons for increased androgens, oligo or anovulation, and polycystic ovaries (11).
Hirsutism is a common disorder that affects 5 - 15% of women of reproductive age and varies based on race and ethnicity (12-14). In addition to PCOS, many medications can also cause hirsutism (15). Approximately, 92% of patients are suffering from this disease based on some studies (16). In many cases, an increased androgen level or accelerated response of target tissues to androgen is responsible for hirsutism incidence. A high androgen level causes hirsutism in more than 80% of cases (12, 13).
Elevated LH relative to FSH release has always been noted in PCOS. However, the pulsatile nature of their release and the lack of specificity are the main reasons for not considering LH/FSH ratio as a PCOS diagnostic criteria (17). Obesity plays an essential role in developing hyperandrogenism, and roughly half of the women with PCOS are obese or overweight (18, 19). Insulin resistance is found in both obese and non-obese PCOS cases. Hyperinsulinemia has a positive correlation with some degree of hyperandrogenism. However, higher androgen levels and prominent insulin resistance have been reported in women with obesity and PCOS (19).
Several studies have investigated the various symptoms of PCOS in Iran. However, the relationship between these signs in affected women has not been thoroughly evaluated. Studying simultaneous symptoms and the relationships and interactions between diverse factors can be the basis for the early diagnosis and treatment of this disease.
2. Objectives
The current study was conducted to find any possible relationship between LH/FSH ratio, body mass index (BMI), hirsutism, and menstrual patterns in women with PCOS.
3. Methods
3.1. Sampling
This descriptive cross-sectional study was performed on 400 women with PCOS in their reproductive age. Irregular menstrual patterns, menstrual cramps, and excess hair were observed in all selected individuals. The BMI was calculated for each person based on Wt (kg)/ht (m2), and their obesity states were classified according to the measurements. Blood samples were taken in order to find serum FSH and LH levels using the radioimmunoassay technique. Next, the mean LH/FSH ratio was acquired for all specimens. Finally, the results were evaluated through correlation analysis and logistic regression.
3.2. Statistical Analysis
Statistical analysis was carried out using the SPSS version 19 (IBM, USA). Following descriptive statistics, data were evaluated by the Mann-Whitney test, Chi-squared test, Spearman’s correlation coefficient (represented as “r”). This correlation reveals the degree of relationship between the variables. The r values are between -1 and +1. If r = +1, there is a perfect linear correlation, meanwhile r = -1 shows perfect inverse linear correlation. When r = 0, the two variables are not correlated at all.
4. Results
The distribution of the clinical presentations of control and PCOS groups is shown in Table 1. According to Table 1, there was no statistically significant difference between the control and PCOS groups in terms of age and height, while the mean weight and BMI of the PCOS group were significantly higher than the control group (P ≤ 0.001). The results of comparing clinical factors between the control group and PCOS cases are summarized in Table 2. As could be seen in Table 2, in this study, the percentage of clinical factors (hirsutism, irregular menstruation, and menstrual pain) in women with PCOS was significantly higher than the control group (P ≤ 0.001). Table 3 indicates the comparison of LH, FSH, and LH/FSH between control and PCOS groups. The findings demonstrated that the mean of LH, FSH, and LH/FSH in women with PCOS was significantly higher than in the control group (P ≤ 0.001).
| Control (N = 500) | PCOS (N = 400) | P-Value b | |
|---|---|---|---|
| Age (y) | 29.96 ± 4.26 | 29.62 ± 5.04 | 0.117 |
| Weight (kg) | 70.55 ± 8.21 | 72.85 ± 9.03 | ≤ 0.001 |
| Height (cm) | 165.27 ± 5.24 | 164.87 ± 5.77 | 0.45 |
| BMI (kg/m2) | 25.68 ± 2.31 | 26.79 ± 2.95 | ≤ 0.001 |
aData are presented as mean ± SEM and were analyzed by the Mann-Whitney test.
bP-value ≤ 0.05 is statistically significant.
| Control (N = 500) | PCOS (N = 400) | P-Value c | |
|---|---|---|---|
| Hirsutism | 292 (58.4) | 356 (89) | ≤ 0.001 |
| Irregular menses | 95 (19) | 358 (89.5) | ≤ 0.001 |
| Menstrual pain | 259 (51.8) | 358 (89.5) | ≤ 0.001 |
aData are presented as No. (%).
bData were analyzed by the chi-squared test.
cP-value ≤ 0.05 is statistically significant.
| Control (N = 500) | PCOS (N = 400) | P-Value b | |
|---|---|---|---|
| LH (mIU/mL) | 6.01 ± 0.59 | 19.08 ± 1.49 | ≤ 0.001 |
| FSH (mIU/mL) | 5.34 ± 0.64 | 6.31 ± 1.46 | ≤ 0.001 |
| LH/FSH | 1.14 ± 0.17 | 3.22 ± 0.93 | ≤ 0.001 |
aData are presented as mean ± SEM and were analyzed by the Mann-Whitney test.
bP-value ≤ 0.05 is statistically significant.
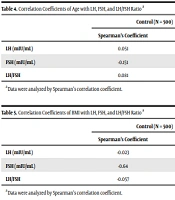
The correlation coefficients of age with LH, FSH, and LH/FSH ratio are shown in Table 4. As could be observed, in this study, Spearman's analysis revealed no statistically significant relationship between LH and age in any of the control and PCOS groups (P ≥ 0.05). Furthermore, age and FSH had weak positive (r = 0.118, P = 0.018) and significant negative (r = -0.151, P = 0) correlations in the PCOS and control groups, respectively. Our results also demonstrated a significant negative relationship between age and LH/FSH in the PCOS group (r = -0.106, P = 0.034). Table 5 shows the correlation coefficients of BMI with LH, FSH, and LH/FSH ratio. According to Table 5, BMI was not significantly correlated with LH, FSH, and LH/FSH in the control and PCOS groups (P ≥ 0.05).
| Control (N = 500) | PCOS (N = 400) | |||
|---|---|---|---|---|
| Spearman’s Coefficient | P-Value | Spearman’s Coefficient | P-Value | |
| LH (mIU/mL) | 0.051 | 0.256 | 0.031 | 0.541 |
| FSH (mIU/mL) | -0.151 | 0.001 | 0.118 | 0.018 |
| LH/FSH | 0.081 | 0.071 | -0.106 | 0.034 |
aData were analyzed by Spearman’s correlation coefficient.
| Control (N = 500) | PCOS (N = 400) | |||
|---|---|---|---|---|
| Spearman’s Coefficient | P-Value | Spearman’s Coefficient | P-Value | |
| LH (mIU/mL) | -0.023 | 0.612 | -0.015 | 0.759 |
| FSH (mIU/mL) | -0.64 | 0.151 | 0.004 | 0.929 |
| LH/FSH | -0.057 | 0.203 | -0.01 | 0.847 |
aData were analyzed by Spearman’s correlation coefficient.
5. Discussion
The PCOS has not been considered a definite typical endocrine disorder with an exclusive foundation or pathophysiology. Different causes, such as genetic and environment, play a role in the pathophysiology of this multi-factorial disease. It could be regarded as an ultimate frequent trail of anovulation (20). The findings of the current study showed that overweight and obesity are common in women with PCOS as 71.5%, 55%, and 16.5% had a BMI > 25, 25 - 30, and > 30, respectively. Only 28.5% had BMI < 25. These values are much higher than those in the study of Pasquali. According to Pasquali, exclusively 35% were dealing with overweight or obesity (19). In addition, Kiddy et al. reported that 50% of women with PCOS in their study were overweight. In the mentioned study, the higher prevalence of overweight and obesity followed by the symptoms of metabolic syndrome resulted from the diet, such as fatty foods and carbohydrates. Most examined women did not have regular exercise or physical activity (21).
As shown in Table 2, we observed that the percentage of clinical factors, including hirsutism, irregular menstruation, and menstrual pain in women with PCOS, was significantly higher than in the control group. These findings are consistent with previous research by Vasheghani F and Alnakash AH. One of the most common symptoms of hyperandrogenism is hirsutism, along with obesity in particular (22). Hirsutism prevalence was 89% among the examined PCOS samples in the present study. This result is quite close to those of Kiddy et al. and Esmaeilzadeh et al., as their results were 73% and 69.2%, respectively (21, 23).
We found that 89.5% of 400 women struggled with irregular menses and menstrual pains. On the one hand, menstrual problems are one of the most common clinical symptoms of PCOS (24). On the other hand, menstrual pain cannot be attributed exclusively to PCOS because menstrual cramps exist in normal women as well. Menstrual pain may happen due to endometriosis, fibroma, pelvic inflammatory disease, premenstrual syndrome, genital tract infection, stress, or even nervousness in every group of women (15).
According to Table 3, in this study, the mean of LH, FSH, and LH/FSH in women with PCOS was significantly higher than in the control group. The results of the present study in this regard are consistent with several other investigations (25, 26). It cannot be said that higher BMI and LH/FSH ratio are essentially related to the greater incidence of PCOS. Sharquie et al. stated that LH/FSH ratio had a diminutive role in PCOS diagnosis (27). Alnakash and Al-Tae'e concluded no significant statistical correlation between LH/FSH ratio and PCOS manifestations. In their study, the mean LH/FSH ratio in women with PCOS was 3.22, which is relatively high (25).
Despite traditional beliefs, it is evident that not all women with PCOS are obese. Moreover, a normal LH/FSH ratio might be observed in PCOS cases. Hormonal and biochemical mechanisms are not the same in all women. Some studies suggest that higher BMI inevitably indicates a higher LH/FSH ratio and greater hirsutism or menstrual disturbance incidence. Insler et al. stated that serum LH was significantly higher in non-obese women with PCOS than obese counterparts (28). Pagan et al. showed an inverse relationship between LH and BMI in women with PCOS (29). Kiddy et al. found an inverse correlation between the mean FSH and BMI in the obese subgroup (21). According to Table 5, no significant correlation was observed between these variables.
5.1. Conclusions
Our results may provide some answers to different aspects of this mysterious syndrome. Traditional concepts were based on the fact that heavier patient has a higher LH/FSH ratio. According to the findings of the current study, unknown aspects of the syndrome are still unsolved, and further studies are required. This study is based on the incidence of metabolic syndromes and hormonal disorders following the onset of PCOS, and genetic studies on polycystic ovary syndrome in Iranian women are not included. It is recommended to investigate genetic principles in future studies.