1. Background
In some countries, medicinal plants are used to control and repel insects and eliminate parasites that cause long-lasting, damaging diseases on humans and animals (1, 2). Based on the World Health Organization (WHO) statistics, approximately 80% of people worldwide tend to apply herbal medicine to solve health problems (3). However, they may also have devastating side effects (4, 5).
Bladder cherry, Physalis alkekengi (PA), belongs to the Solanaceae family with various therapeutic properties. Anti-viral, anti-inflammatory, analgesic, and anti-bacterial properties of PA have been investigated. The anti-fever properties have also been reported (6). Phytochemical studies have demonstrated that PA contains alkaloids, polyphenol compounds (like ascorbic acid), flavonoids, and vitamin C, which its therapeutic impacts may be due to the presence of these compounds (7).
In Taiwan, PA is used as a topical medicine in the treatment of tumors. Their diuretic, laxative, and anti-inflammatory properties have also been studied by the Middle East and Indian researchers (8, 9). Their analgesic properties are used for rheumatism, sore throat, and gastrointestinal pain (5).
In India, its leaves are used to treat viral hepatitis and gonorrhea, while its fruits are used to treat urinary system diseases and gout (10). In Guatemala, this plant is used to treat gonorrhea and sleep problems (4). Anti-tumor properties of PA, which act through the inhibition of topoisomerase II enzyme, were reported by Chen in 1992 (9). Moreover, a recent investigation indicated that PA could prevent the growth of human non-small-cell lung cancer cells by arresting the cells at the G2/M phase of the cell cycle (11). The effect of aqueous extract of PA fruit on the estrous cycle, reproduction, and creatine kinase enzyme (Ck-BB isozyme) in mouse uterus was investigated (12). These researchers concluded that this enzyme causes 100% distrusts and reduces the number of babies. In 2004, this group reported that an aqueous extract of PA reduced the activity of uterine glucose-6-phosphate dehydrogenase (G6PD) (estrogen-induced proteins) by 25% (13). Shekar-Foroosh et al. assessed the effects of PA extract on thyroid hormone and indicated that its injection at high doses increased TSH levels in mice serum (14).
2. Objectives
According to the extensive usage of PA extract in traditional medicine, determining possible side effects is of great importance. In reviewing the studies conducted on the effects and uses of the PA plant, it was observed that no study had been conducted to evaluate the effect of aqueous extract of this plant on heart and aorta tissues. In this study, the effects of aqueous extract of these plants on the heart and aorta tissues and changes in cardiac enzymes of mice were investigated in BALB/c mice.
3. Methods
3.1. Preparation of Physalis alkekengi Aqueous Extract
Fruit of the PA was collected, dried, and prepared for injection. Then, 30 g of the dried fruit's powder was prepared by the electric mill to which 200 mL of distilled water was added. The resulting suspension was slowly boiled for an hour and filtered with an ordinary paper filter. It was filtered again using Whatman paper filter No. 4 (Sigma-Aldrich, USA) and condensed at temperatures up to 60°C so that the final volume reached 20 mL containing 1.5 g/mL of powdered extract.
3.2. Animals and Experimental Design
In this study, 50 female BALB/c mice, 10 - 12 weeks old, were purchased from the Animal Center of Pasteur Institute (Tehran, Iran). They were divided into five experimental groups as follows (n = 10 each): control, sham, and three experimental groups and maintained under similar conditions with a 12-hour dark/light cycle and free access to standard rodent chow and water. Animal care and processing were performed in conformity with the guidelines of animal experiments by the Ethics Committee of Islamic Azad University (Code of Ethics: IR.IAU.K.REC.1396,75). Animals were kept under Guide for the Care and Use of Laboratory Animals (8th edition, National Research Council).
The experimental groups received three doses of aqueous extract, including 7 g/kg, 15 g/kg, and 19 g/kg by an intraperitoneal (IP) injection for four weeks. The sham group was injected with distilled water, and the control group received no injection. Also, 48 h after the last injection, mice were euthanized by carbon dioxide (CO2) narcosis, and serum and heart samples were collected for further investigation.
3.3. Investigating Serum Levels of LDH and CPK
Blood samples were taken to measure lactate dehydrogenase (LDH) and creatine phosphokinase (CPK). Next, blood samples were allowed to clot, and serum was separated using centrifugation at 4000 rpm for 5 minutes at 4°C. Then, the Hughes method and creatine kinase myocardial band (CPK-MB) test were used to measure LDH and CPK.
3.4. Aorta Measurement and Histopathological Assessment
After removing the hearts and aortas from the dead mice, the aorta diameter for all groups was measured accurately using a caliper. The heart tissues were immediately sectioned. Next, the excised hearts from experimental groups were fixed in 10% paraformaldehyde and embedded in paraffin. Sections were stained with hematoxylin-eosin (H&E) staining to investigate the alternate heart histological features.
3.5. Statistical Analysis
The obtained data were analyzed using SPSS software by one-way analysis of variance (ANOVA) and Tukey‘s and Duncan's tests. The data are presented as the mean ± standard deviation (S.D), and a P-value ≤ 0.05 indicated a statistically significant difference.
4. Results
4.1. Serological Investigation
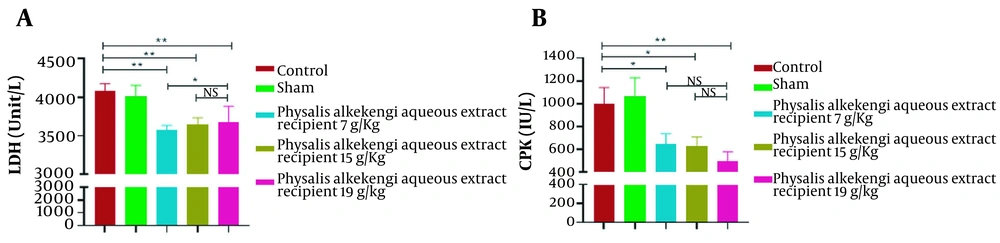
Injection of PA aqueous extract at different doses of 7, 15, and 19 g/Kg caused a significant decrease in the LDH enzyme in the experimental groups compared to the control and sham (P-value < 0.001) groups. Also, injection of PA aqueous extract at a dose of 19 g/Kg significantly reduced LDH enzyme compared to the experimental group receiving 7 g/Kg of PA aqueous extract (P-value < 0.01) (Figure 1A).
Serological investigation of different experimental groups. (A) Lactate dehydrogenase (LDH) measurement. (B) Creatine phosphokinase (CPK) measurement. The data are represented as the mean values ± SD (n = 10 in each group). Statistical data analysis was performed with the One-way ANOVA. NS; not significant; * P < 0.05 and ** P < 0.001.
On the other hand, injection of PA aqueous extract at different doses of 7, 15, and 19 g/Kg caused a significant reduction in CPK enzyme compared to the control and sham groups (P-value < 0.0001). However, no significant difference was observed between the experimental groups (Figure 1B).
4.2. Macroscopic Investigations of Heart Tissues
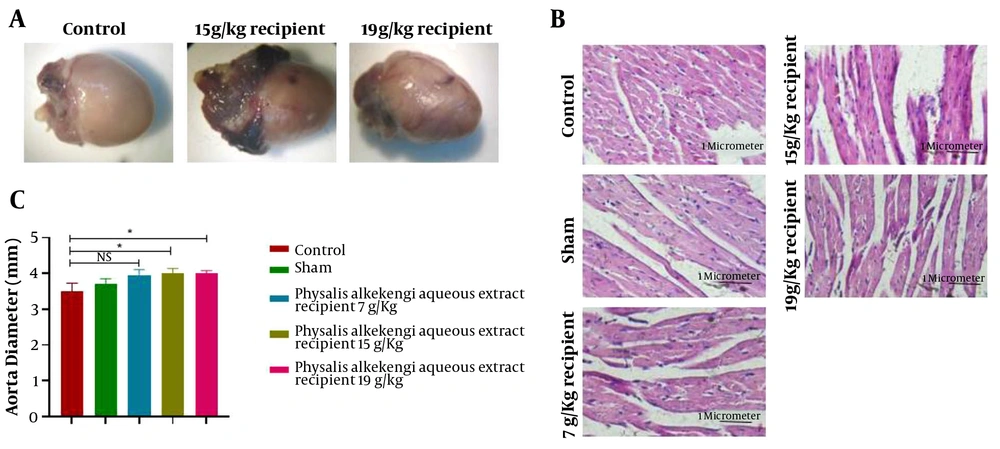
Regarding heart tissues, it was observed that the tissues of the experimental groups, demonstrated swollen blood vessels and more significant congested aorta compared to the control and sham groups. This observation was more evident in groups exposed to higher doses of PA aqueous extract (Figure 2A). Additionally, there was a significant increase in the aortic diameter of the experimental groups receiving 15 g/Kg and 19 g/Kg doses of the PA aqueous extract compared to the control group (P-value < 0.01).
H&E sections, heart tissues, and aortal diameter of the experimental groups. (A) The macroscopic evaluation of heart tissues. (B) H&E staining with 400x magnification (C) Aortal diameter measurement. The data are represented as the mean values ± SD (n = 10 in each group). Statistical data analysis was performed with the One-way ANOVA. NS; not significant, and * P < 0.05
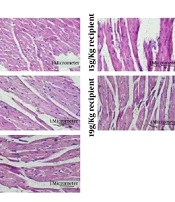
4.3. Histopathological Investigation by H&E Staining
A microscopic view of cardiac tissue sections is shown in Figure 2B. In all experimental groups, blood vessels were smaller, and the chance of the presence of eukaryotic nuclei was lower than the control and sham groups. This decrease appeared to be more visible with the increase of dose. Moreover, the increase in interstitial spaces was illustrated in all experimental groups compared to the control and sham groups, with being more significant by increasing the PA dose. Stepped lines in the experimental groups were indistinguishable, indicating increased myocyte injury and inflammation.
5. Discussion
Human use of medicinal plants against diseases, repel insects, and parasites that harm humans have a long history. Nowadays, more than 80% of people apply herbs for their therapeutic approaches. In addition, many chemical drugs are modeled on herbal substances (15). The use of medicinal plants should be very prudent because many of them have not been assessed for purity and potency and may contain some substances that result in adverse side effects (16).
PA is a herbaceous plant with the potential of treating urinary tract diseases, gout, and rheumatism due to its anti-inflammatory, anti-bacterial, analgesic, laxative, diuretic, and antimitotic properties. Recent investigations have demonstrated that PA positively impacts some cancers, thyroid hormones, liver enzymes, and sexual and reproductive hormones (17-20).
In this study, the effects of PA aqueous extract were investigated on the heart tissue and aorta of adult female BALB/c mice at three different doses of 7, 15, and 19 g/Kg.
Investigation of aortic diameter revealed that PA aqueous extract caused a significant increase in aortic diameter compared to the control group. It was also observed that the experimental groups, compared to the control group, had more swollen and apparent blood vessels and more congested and enlarged aorta. In the microscopic examination, increased interstitial spaces reduced blood vessels and eukaryotic nuclei, and unrecognizable stepped lines were indicated compared to the control group. Similarly, another investigation by Perk et al. revealed that treatment of Wistar rats with lyophilized fruit juice of Physalis peruviana L (PPL) resulted in cardiac toxicity by increasing the cardiac troponin and serum potassium levels in male Wistar rats but had no significant impacts on female Wistar rats (21).
On the other hand, our research showed that PA aqueous extract caused changes in LDH and CPK as cardiac enzymes; thus, it can be concluded that this extract caused damage to the heart. Tissue damage to the heart and aorta indicates that LDH and CPK enzymes are first increased and caused degradation in the tissue; then, considering two days of rest after the final injections, the levels of these enzymes fell below normal. This negative impact was more remarkable at high doses. Vesal et al. assessed the antagonistic estradiol effect of aqueous extract of PA fruit on the brain and showed that the aqueous extract might inhibit the release of gonadotrophin hormones of the hypothalamus and release of luteinizing hormone of the hypothalamus (13). According to another investigation by Vessal et al. on the effect of aqueous extract of PA fruit on the estrous cycle, reproduction, and creatine kinase (Ck-BB isozyme) in rat's uterus caused 100% production of diestrus, reduction of the number of babies, and plasma progesterone. Also, the activity of creatine kinase 1BB in the uterus was inhibited time-dependently (12).
Additionally, Montaseri et al. assessed the effect of alcoholic extract of PA on female rats and showed that this extract has a negative effect on fertility and ovulation in female rats (22). Javdan and Estakhr showed that using an aqueous extract of PA can negatively impact the growth of embryos and differentiation of some cells (23). The results of our research indicated that due to the reduction in cardiac enzymes and blood vessel swelling after the injection of PA aqueous extract, it is possible that this extract can harm embryonic enzymes and the differentiation of cells. Moreover, an investigation by Shekar-Foroosh et al. to evaluate the effect of PA aqueous extract on the thyroid hormone suggested that its injection at high doses causes an increase in thyroid-stimulating hormone (TSH) serum levels (14).
Although PA aqueous extract has anti-bacterial, anti-parasitic, anti-fungal, and anti-cancer properties, its administration in female BALB/c mice showed adverse effects on the heart and aorta with a significant reduction in LDH and CPK enzymes.
5.1. Conclusions
Although the PA has been used as a beneficial herbal medicine in Iran and other countries, it should be considered that its use may have adverse impacts. In this study, we demonstrated that the aqueous extract of this plant could have a detrimental effect on the heart, aorta, and cardiac enzymes. To be more precise, further investigations on molecular mechanisms are suggested.