1. Background
Organophosphates insecticides, such as chlorpyrifos (CPF), have been widely used as insecticides around the world. CPF is also frequently used in agriculture; therefore, it can be easily found in water, food, and air. CPF can be accumulated in humans and distributed throughout the body (1). Moreover, animal experiments demonstrated some neurotoxic effects of CPF exposure. Animals also showed cognitive impairments in the open-field, visual signal detection, Morris water maze, 16-arm radial, and T-maze tasks (2).
The hippocampus is directly or indirectly involved in learning and memory. Moreover, neurodegeneration often leads to the loss of cognitive functions and dementia. Studies have reported that injuries to cerebral and hippocampal neural cells are critical to inducing cognition impairment. Besides, programmed cell death has been proposed as a reason for neurodegeneration (3, 4). Uncontrolled neural apoptosis events in the brain, specifically in the cortex and hippocampus, promote learning and memory impairment (5). Studies have documented that the overproduction of reactive oxygen species (ROS) by brain mitochondria is a key factor in triggering apoptosis in the brain (6).
Overproduction of ROS or low levels of antioxidants can result in protein oxidation, lipid peroxidation, and mitochondrial DNA damage, leading to the dysfunction of many biological events (7).
The caspase gene family, including caspase-3, is involved in several important apoptosis pathways and also proteolytic events in neuronal cells. Caspase-3 has both apoptotic and non-apoptotic roles and is a regulatory molecule in neurogenesis and synaptic activity in neural differentiation (8, 9).
Findings are limited about the impacts of CPF on apoptotic alterations and cognitive functions in the brain, specifically the hippocampus (10-12). CPF can affect the antioxidant capacity and ROS production. Studies have documented that CPF exposure caused events such as low antioxidant capacity, high ROS levels, and activation of apoptosis cascades. These factors are involved in the development of some neurodegenerative diseases (13).
Previous studies have reported different behavioral tests to determine learning and memory function; thus, it is hard to study repeated CPF exposure effects on cognitive functions. In this line, subjects in studies showed conflicting behaviors regarding the effects of CPF exposure (14-18). In this regard, it is necessary to pay more attention to the effects of CPF on different types of memory, such as working memory, and biochemical mediators, such as caspase-3.
2. Objectives
Our study was designed to evaluate the chlorpyrifos effects on passive avoidance memory and caspase-3 alterations in the hippocampus.
3. Methods
3.1. Animals
Male Wistar rats (Rattus norvegicus, 180 - 220 g) were provided from the Pasteur Institute of Iran. Animals were placed in cages (four animals per cage) with available food and water and under physiological lighting) 12-h light/dark cycle). The animal house temperature was set as 23 ± 3°C. In all experiments, the Laboratory Animal Care Principals were followed (NIH guideline, No. 80-23, Updated1996). The study protocol was approved by the bioethical board of the Islamic Azad University, Central Tehran Branch, Tehran, Iran. This project is a part of a larger project (Iriau.iaug.rec1398.008).
3.2. Chemicals
CPF [CAS No. 2921-88-2] was provided from Sigma-Aldrich Co. (St. Louis, MO, USA), and dimethyl sulfoxide (DMSO) was purchased from Merck (Darmstadt, Germany). Initially, CPF was dissolved and diluted in dimethyl-sulfoxide (DMSO) and 0.9% normal saline (the final DMSO concentration was 0.01% v/v).
3.3. Animal Groups
Animals were randomly divided into three groups, including control, sham, and CPF groups (eight in each). The control group did not receive the intervention. Animals in the CPF group received intraperitoneal CPF (3.0 mg/kg) for five days per week (single daily injection) for two consecutive weeks, while the sham group received the vehicle with the same volume of DMSO diluted in 0.9% normal saline. The CPF volume for injection was selected according to our pilot study results (data are not presented in this study) and Yan et al.’s study, based on the highest toxicity and the lowest animal death (2). In the experimental group, animals received CPF for two weeks (five days per week), and the passive avoidance test was done after a one-week treatment. At the end of the experiments, animals were sacrificed, and the hippocampus was removed for western blot analysis.
3.4. Passive Avoidance Test
Short- and mid-term memory was evaluated by the passive avoidance behavioral task. The shuttle box included two compartments with similar dimensions (light or dark side; 20 × 80 × 20 cm). There was a movable door (7 × 9 cm) between the light and dark rooms. The dark room was floored with stainless steel rods. Electric shocks with the frequency of 50 Hz, length of 1.5 s, and intensity of 1.5 - 2 mA were delivered through a stimulator. After the habituation session, each rat was placed in the light room, and the door was opened after 5 s so that the subject could move freely in the dark or light room. When the rat entered the dark side, the door was immediately closed, and the rat received an electrical shock for 3 s. After 24h, the subject was placed in the light chamber, and the latency to enter the dark chamber was documented. Animals that avoided the dark room for a maximum of 5 min were considered with acceptable avoidance memory.
3.5. Western Blot Procedure
The RIPA buffer (radioimmunoprecipitation assay buffer) was used to homogenize hippocampus [Triton X-100 (1%), Tris–HCl (0.01m), and pH 7.4, NaCl (0.15 m), EDTA 19 (ethylene diamine tetraacetic acid;10 - 03 m), SDS (Sodium Dodecyl Sulfate; 0.01%), and protease inhibitor cocktail (0.1%)]. The homogenates were centrifuged for 10 min at 4°C (10,000 × g). Collected samples were used for protein detection. Total protein extracts were treated in SDS buffer [mercaptoethanol (10%), glycerol (20%), Tris–HCl (0.125 m,) and SDS (4%), pH: 6.8]. Then, the samples were exposed to SDS-polyacrylamide gel-electrophorese, immunoblotting with polyclonal antibodies of rabbit against procaspase-3, cytochrome C, and beta-actin (1:1000 dilutions), and Horseradish peroxidase-conjugated secondary antibody (1: 10,000 dilutions). All proteins were identified through Luminol (Santa Cruz).
3.6. Statistical Analysis
The results were analyzed by SPSS (version 20) using one-way ANOVA followed by Tukey post-hoc test. The results are reported as mean ± SD, and significance was considered at < 0.05.
4. Results
4.1. Caspase-3 Analysis
Figure 1 represents the hippocampal caspase-3 levels in all experimental groups. There were no significant differences between the control and sham groups. Therefore, drug solvents did not affect caspase-3 levels. However, caspase-3 significantly increased in the CPF group compared with the control and sham groups (both P < 0.001).
4.2. Behavioral Results
The data of passive avoidance experiments in all groups are represented in Figure 2. There were no significant differences between the groups in the latency to enter the dark room 24h and one week after receiving an electrical foot shock. Also, the CPF group significantly spent more time in the dark room compared to the control and sham groups, 24h and one week after receiving the foot shock (P < 0.01 and P < 0.05, respectively). Our results showed that short- memory and mid-term memory consolidation was impaired by CPF because latency presents memory and the dark room stay time shows memory consolidation.
The effects of chlorpyrifos (CPF) on passive avoidance test. A, Latency to enter the dark room 24 hours and one week after receiving the foot shock) (** P < .01, significant difference between the control and CPF groups; † P < .05, significant difference between the sham and CPF groups); B, Time spent in the dark room 24 hours and one week after receiving the foot shock [*** P < 0.001, significant difference between the control and CPF groups; † P < 0.05, significant difference between the sham and CPF groups; The data are shown as mean ± SD (n = 8 in each group)].
5. Discussion
This study investigated the harmful impacts of CPF on short- and mid-term memory and caspase-3 levels in the hippocampus. The results of this study revealed that CPF exposure impaired short- and mid-term memory in the CPF group compared to the control group.
Although human and animal studies have reported some cognitive dysfunctions, such as memory and attention impairment, anxiety, and other mood disorders after exposure to CPF (19, 20), more research is still needed. In the present study, intraperitoneal CPF injections (3.0 mg/kg) for five days per week for two consecutive weeks led to retrieval memory impairment 24 h and one week after receiving foot shock in CPF-treated rats.
Previous studies have reported conflicting results on the effects of using different doses of CPF and durations of treatment on motor activity, behavioral tasks, and memory in animals. For example, repeated exposure to CPF at 40 mg/kg for four days caused no motor activity impairment, while exposure to CPF at 0, 1, 3, and 10 mg/kg for four weeks and 15 mg/kg for four weeks led to motor activity dysfunction (14, 15, 18). Moreover, 0, 1, 3, and 10 mg/kg of CPF for four weeks and 1 and 5 mg/kg for one year caused no measurable impairments in attention, learning, and memory in delayed matching to position task, while repeated oral administration of CPF for eight weeks (12.5 mg/kg/5 days) caused learning impairment (16) and spatial memory impairment (17). In this line, our results showed that CPF treatment (3.0 mg/kg/5days) for two weeks impaired passive avoidance memory task. The conflicting results appear to be due to the use of different doses, treatment duration, and different behavioral tasks in the studies because different neurobehavioral processes are influenced by the type of behavioral tasks.
Previous studies have demonstrated hippocampal neurodegeneration as the main cause of learning and memory impairment in neurodegenerative diseases (21). They have also proposed that apoptosis is involved in hippocampal neuronal cell death (3). It seems that apoptosis and related neuronal cell loss have major roles in cognitive dysfunction and memory impairment. In this regard, CPF induced apoptosis in embryonic and newborn cortical neurons of rats (22).
Apoptosis is necessary for the development of nervous system for postmitotic neurons and neuronal precursor elimination (23), and caspases-3 is a key effector in cell apoptosis regulation (24, 25). To confirm the involvement of caspases-3 in CPT-induced apoptosis, we assessed its level in the hippocampus. In the present study, the CPF-treated group was found with increased caspase-3 levels in the hippocampus, suggesting that CPF enhanced apoptosis in the hippocampus. In line with this, it has been revealed that caspase-3 is one of the critical proteases in self-activating, anti-Fas, and staurosporine-related apoptosis (26). In some apoptotic pathways, caspase-3 is activated by cytochrome c, and ROS also can induce cytochrome c release. Therefore, it seems that a chain of events, including caspase-3 activation, cytochrome c release, and ROS production, contribute in apoptosis (26).
5.1. Conclusion
In summary, the present study showed that CPF exposure (3 mg/kg for five days) for two weeks caused short- and long-term memory consolidation in rats, while it did not affect latency. Also, caspase-3 levels increased in CPF-treated rats, which is involved in cell apoptosis events and neurodegeneration. It seems that caspase-3 plays a role in memory impairment; however, more investigations are needed.
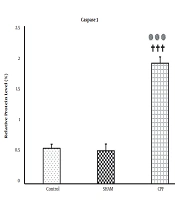
![The caspase-3 levels in the experimental groups [*** P < 0.001, significant difference between the control and CPF groups; ††† P < .001, significant difference between the sham and CPF groups; The data are shown as mean ± SD (n = 8 in each group)]. The caspase-3 levels in the experimental groups [*** P < 0.001, significant difference between the control and CPF groups; ††† P < .001, significant difference between the sham and CPF groups; The data are shown as mean ± SD (n = 8 in each group)].](https://services.brieflands.com/cdn/serve/3170b/896cb8bf6df27e0f8b4463f8d2733b6edfc3dc7d/thrita-123356-i001-F1-preview.webp)
![The effects of chlorpyrifos (CPF) on passive avoidance test. A, Latency to enter the dark room 24 hours and one week after receiving the foot shock) (** P < .01, significant difference between the control and CPF groups; † P < .05, significant difference between the sham and CPF groups); B, Time spent in the dark room 24 hours and one week after receiving the foot shock [*** P < 0.001, significant difference between the control and CPF groups; † P < 0.05, significant difference between the sham and CPF groups; The data are shown as mean ± SD (n = 8 in each group)]. The effects of chlorpyrifos (CPF) on passive avoidance test. A, Latency to enter the dark room 24 hours and one week after receiving the foot shock) (** P < .01, significant difference between the control and CPF groups; † P < .05, significant difference between the sham and CPF groups); B, Time spent in the dark room 24 hours and one week after receiving the foot shock [*** P < 0.001, significant difference between the control and CPF groups; † P < 0.05, significant difference between the sham and CPF groups; The data are shown as mean ± SD (n = 8 in each group)].](https://services.brieflands.com/cdn/serve/3170b/31a195367f061bc1eebd10887172575751922b9b/thrita-123356-i002-F2-preview.webp)