1. Context
Osteoporosis is the most common metabolic bone disease characterized by decreased bone strength (1). In this condition, the sufferer is prone to an increased risk of fractures. Genetic and environmental agents such as diet, sedentary lifestyle with too little exercise, heavy smoking, exposure to the toxic metal cadmium, ovariectomy, and obesity affect the process of osteoporosis. The incidence of osteoporosis depends on both age and sex. It is most common in females, especially after menopause (2). In fact, the pathogenesis of osteoporosis is complex and is influenced by a variety of factors (1). Bone is one of the tissues that need daily mechanical stress to maintain its natural structure. Decreased physical activity (PA) may lead to decreased bone mineral density (BMD) and increased fracture rate (3). Studies show that regular PA is one of the most effective and least costly non-pharmacological methods for the prevention and control of osteoporosis (4). The PA transmits mechanical force to the bone and stimulates intracellular processes in bone tissue. The mechanical load (something which exerts an opposing force on the system; hence system needs more power to perform that work) on the bone activates a set of signaling pathways in the bone cell that increase the deposition of minerals in the bone, resulting in increased BMD in the bone tissue and consequently maintaining its strength (5). Since mechanical load is an important factor in maintaining BMD, many studies have examined the effect of various exercise programs on BMD and its structural features (6, 7). Physical activity increases BMD through activating anabolic processes and increasing mineral content (8). Bone mineral density has been shown to increase by up to 20% in areas of bone subjected to mechanical stress (9, 10). In general, high mechanical loads, as well as frequent loading (such as resistance exercises or exercises with weights and ropes) increase bone mass, while activities that put less pressure on the bone, even if applied for longer periods, have a negligible effect on BMD (11, 12). Such as resistance exercises or exercises with weights and ropes.
A review of the existing literature on PA and BMD showed that several studies had been conducted in this field. In this regard, Iranian researchers have conducted various studies with many types of training protocols on both human and animal subjects. Accordingly, a review of research has been conducted by Iranian researchers on BMD.
2. Physical Activity and Bone Density
2.1. Human Studies
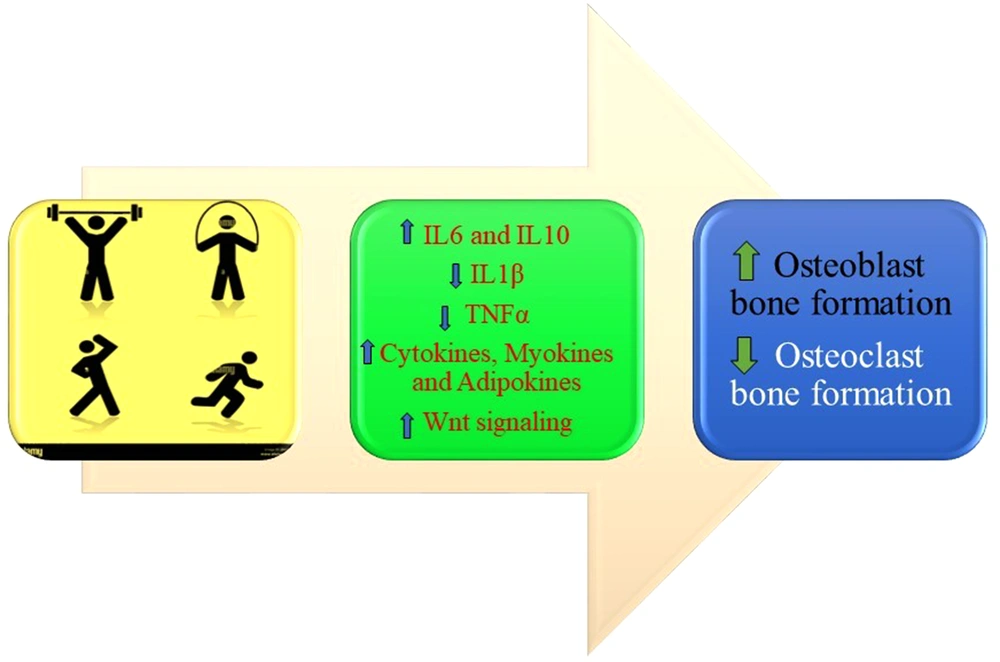
The PA positively affects bone metabolism via different mechanisms, such as activation of an inflammatory cascade, triggering an immunological response due to enhanced IL-6, and stimulation of Wnt signaling pathway (13) (Figure 1).
Cross-sectional studies generally compare the BMD of athletes to sedentary individuals or different levels of PA related to bone mass. The results of these studies report higher BMD associated with higher levels of PA (14, 15). Recent studies have focused on the relationship between BMD, body composition, and lifestyle (16, 17). For this reason, researchers have examined the effectiveness of PA on bone characteristics. A review of the research literature showed that exercise types, especially weight-bearing exercises such as resistance exercises, exercises with weights and ropes, jogging, jumping rope, step aerobics, climbing stairs, dancing, hiking, and elliptical training machines, have a greater effect on bone density. In this regard, Rahimian and Hejazi studied the comparison of femoral and radial BMD in elite female athletes in non-weight-bearing (swimming) and weight-bearing (gymnastics, tennis, and table tennis) disciplines (18). The subjects of this study included 23 members of the national women’s team aged 20-25 years. Bone mineral density was assessed using the DEXA method in two areas of the body (femoral neck and radius) and was compared with Iranian and world standards. The results showed that long-term training with weight-bearing exercises increased femoral density compared to non-athletes in Iran and the world (18). Also, non-weight-bearing training, such as swimming, had a lesser effect on bone density than weight-bearing training (18). Generally, the effect of various sports training on bone density depends on the mechanical stress induced in the bone (18).
Jafarzadeh et al. examined the prevalence of osteopenia and osteoporosis in professional swimmers (19). Lumbar mineral depletion of 13 elite swimmers and 13 non-athletes was measured by a DEXA and according to the relevant norms (19). The main result of this study showed that 61.5% of elite swimmers had osteopenia in the lumbar region, while only 38.5% in the control group were categorized for osteopenia in this region (19). Since mechanical stresses on the bones are caused by muscle tension and ground reaction forces, this second factor seems to play a more effective role in the development and increase of BMD. The researchers believe that elite swimmers typically practice between three and 3.5 hours a day. As a result, they are less in contact with the ground for three to 3.5 hours a day and receive less ground reaction force than their non-athlete subjects. On the other hand, strength and short-term exercises seem to have a greater effect on bone density development than non-weight-bearing endurance exercises such as swimming. Therefore, swimmers are advised to engage in activities such as running and jumping, in addition to their professional activities, to prevent a decrease in BMD. Ghasemi et al. investigated the effect of water exercise on bone density in premenopausal women (20). Twenty premenopausal women aged 40 - 45 years were assigned to water exercise (n = 10) or control group (n = 10). The water exercise group performed 12 weeks of water training three times a week, and each session lasted 70 minutes. Femoral shaft and neck mineral density was measured before and after the 12 weeks by a DEXA (20). Bone mineral density in the water exercise group was significantly higher than in the control group. The researchers found that weight-bearing exercises in water were recommended for premenopausal women because they prevented a decrease in BMD due to aging during this period (20). In this regard, Hashemi et al. examined the effect of 12 weeks of water resistance training on bone density in elderly women (60 - 75 years old) (21). Twenty-four elderly women were selected after medical screening and randomly assigned to water resistance and control groups. Bone density (femoral neck and lumbar vertebrae) was assessed pre- and post-training via DEXA. The subjects in the experimental group performed a water resistance training program (12 weeks, two sessions per week, one hour per session) (21). The control group did not receive any intervention. The results showed that 12 weeks of water resistance training had no impact on the BMD in the experimental group (21). However, the BMD decreased in the control group (21). According to this study, 12 weeks of water resistance training seems to maintain bone density in older women (21). In confirmation of these results, Barzanjeh et al. examined the effect of 12 months of strength training in water on the bone density of the lumbar vertebrae and femur in postmenopausal women with osteoporosis (22). Thirty postmenopausal women with osteoporosis aged 50 - 65 years were assigned to experimental and control groups (22). The experimental group performed 12 months of water-based strength training, three sessions per week (Monday, Wednesday, and Friday), and averaged 50 minutes per session (22). Bone density was measured by X-ray energy uptake and standard method for the L2 and L3 vertebrae of the lumbar spine and femoral neck. The results showed that 12 months of water-based strength training increased bone density of the L2 and L3 vertebrae, and femoral neck of postmenopausal women (22). Strength training in the water had a significant effect on the percentage of T score (a T-score is a standard deviation; a mathematical term that calculates how much a result varies from the average or mean) of the L2 and L3 vertebrae of postmenopausal women (22). Based on this study, strength training in water can possibly lead to increased bone density in postmenopausal women with osteoporosis (22). These results were supported by Movassagh Behestani and Tofighi (23). The effect of aerobic training in water on BMD was evaluated in obese postmenopausal women. Twenty obese menopausal women were randomly divided into experimental and control groups. The experimental group performed water-based aerobic training for 12 weeks. The duration of each training session was 90 minutes, and they performed at 65 - 75% of their maximum heart rate in water, while the control group did not do any exercise training (23). Blood samples were collected to measure the levels of parathyroid hormone, calcium, and phosphorus. Bone density was measured before and after the training period. Femoral BMD, parathyroid hormone concentration, and plasma calcium concentration increased in the water-based aerobic exercise group with no change in the control group. According to Movassagh Behestani and Tofighi, aerobic training in water improved bone density in the femur of obese postmenopausal women (23). In this regard, Vanaky et al. investigated the effect of weight-bearing aquatic exercise on the bone density of the lumbar vertebrae of overweight women aged 50 - 70 years (24). Twenty women were randomly divided into two experimental and control groups (24). The weight-bearing aquatic exercise was performed in the experimental group for 12 weeks, three sessions per week (24). The intensity of the exercise started at 60% of the maximal heart rate and increased to 80% in 12 weeks (24). The duration of each exercise session started from 60 minutes and increased to 90 minutes by the end. The control group did not have an exercise training program during this period. Both groups performed bone density tests before and after training. There was an increase in bone density between pre- and post-training in the experimental group and no change in the control group. Weight-bearing aquatic exercise for 12 weeks increased bone density of the lumbar vertebrae of overweight women aged 50 - 70 years (24). Zaravar et al. examined the effect of aerobic aquatic exercise with restricted blood flow (BFR) on the concentration of growth hormone (GH), insulin-like growth factor-1 (IGF-1), and BMD of elderly women (25). Thirty non-athlete women volunteered and were randomly divided into three groups: Control, BFR aerobic aquatic exercise, and aerobic aquatic exercise. Both groups performed aerobic aquatic exercises (eight weeks, three days per week, one hour per session) (25). The cuff pressure in the training group with BFR was 110 to 220 mmHg. Blood sampling was performed 24 hours before the first and after the last training session (25). The results showed that the training groups increased BMD, IGF-1, and T-score compared to the control group (25). Also, the post-test results of the training group with BFR in comparison with the training group without BFR increased in BMD, GH, IGF-1, and T-score (25). These results demonstrated that water-based exercise in elderly women improved the effect of anabolic hormones in preventing a decrease in bone density, but an exercise in the water with BFR (probably because of the greater effectiveness of hormones) had more beneficial effects on increasing BMD. Therefore, it is recommended to use these exercises in the training program for older women (25).
The effect of aerobic, resistance, and combination training on land on bone density has also been investigated. Akouchakian et al. studied the effect of combined exercise on the BMD of postmenopausal women with cancer (26). Twenty-nine women with breast cancer who underwent surgery, chemotherapy, radiation therapy, and underwent hormone therapy were assigned to the training group and control group (26). Subjects in the experimental group had 15 weeks of exercise, including walking (two sessions per week) and resistance training (two sessions per week, different from walking days). Subjects in the control group did not participate in any exercise program. Body weight, body mass index (BMI), peak oxygen uptake (VO2peak), and femoral and lumbar vertebrae density were measured in both groups before and after 15 weeks. In the experimental group, body weight and BMI decreased, and VO2peak increased, and there was a difference between the two groups in BMD for the lumbar vertebrae but not the femur (26). Malandish et al. studied the effect of 12 weeks of moderate-intensity aerobic training on the BMD of the lumbar and cervical vertebrae as well as serum calcium and phosphorus levels in postmenopausal women (27). Twenty sedentary healthy postmenopausal women were randomly assigned to two groups: exercise (n = 11) and control (n = 9). The exercise group performed 12 weeks, three sessions per week, and 50 - 60 minutes of aerobic walking and jogging at 65 - 70% of the maximum heart rate, but the control group did not participate in an intervention (27). Before and after 12 weeks, BMD of lumbar vertebrae and femoral neck and serum calcium and phosphorus levels were evaluated (27). After 12 weeks of moderate-intensity aerobic exercise, no difference was found in lumbar vertebrae and femoral neck bone density, T-score, and serum calcium and phosphorus levels (27). The findings indicated that a 12-week moderate-intensity aerobic exercise program, such as light walking and jogging, had no effect on bone density and serum calcium and phosphorus levels (27). Aboutalebi et al. examined the effects of 16 weeks of Pilates exercises on BMD in postmenopausal women (28). Thirty postmenopausal women participated and were randomly divided into control and exercise groups. The exercise group performed Pilates exercises for 16 weeks. Before and after 8 and 16 weeks of training, T-score, L1 - L4 lumbar, and femoral neck density were measured. The results showed that the interaction between the groups and across time was significant. Lumbar vertebral bone density had no change after eight weeks of Pilates training compared to before training, but 16 weeks after training BMD increased compared to eight weeks after training. Also, this density after 16 weeks of training compared to before training showed a significant increase in interaction with the control group. Similar results were seen in femoral neck density with lumbar vertebrae. It seems that performing Pilates exercises by postmenopausal women has an effect on BMD and the effectiveness of the training depends on the length of the training program. Therefore, Pilates can be recommended as a suitable training method for postmenopausal women (28). Ahmadi Kakavandi et al. examined the effect of six months of low-intensity, high-volume resistance training (body pump) on bone density and balance in postmenopausal women (29). Twenty-two postmenopausal women participated and were randomly assigned to two groups: resistance training (n = 12) and control (n = 10). The body pump group performed the exercises three times a week for six months, while the control group did not exercise (29). DEXA BMD was measured at the beginning and after six months of training. The results showed that lumbar vertebrae bone density and balance in the resistance training group increased significantly (29). However, no effects were observed on bone density of the femoral neck and forearm after six months (29). Sheykholeslami Vatani and Rezaei examined the effect of eight weeks of resistance training on BMD in postmenopausal women with type 2 diabetes (30). Twenty postmenopausal women with type 2 diabetes participated and were randomly divided into experimental and control groups (30). The experimental group performed resistance exercises for eight weeks, three sessions per week, at 70% of a maximum repetition (30). The control group did not have any regular exercise during this period, but they were similar to the experimental group in terms of dose and medication. To determine BMD, densitometry was assessed in the femurs, distal forearm, and lumbar vertebrae (30). After eight weeks, no change in BMD was observed in the femoral neck, lumbar vertebrae, and forearm bone in exercise and control groups. These findings indicate eight weeks of resistance training has no effect on bone density in postmenopausal women with Type 2 diabetes (30). Mofidi Sadr et al. investigated the effect of 12 weeks of combined training on BMD and some blood parameters in obese and overweight postmenopausal women (31). Twenty-nine inactive postmenopausal women aged 46 - 58 years participated. Participants were divided into combined training (resistance-aerobic) and control (without training) (31). Before and after the 12-week training period, weight, BMI, fat percentage, lumbar and femoral neck bone density (DEXA method), serum calcium, and phosphorus were measured. The training protocol included upper and lower body resistance training for three sets of 8 - 12 repetitions and aerobic training, including running and jump rope intermittently three times per week (31). The experimental group demonstrated an increase in serum calcium, lumbar vertebrae, and femoral neck density compared to the control group, while serum phosphorus did not change. There was a decrease in body weight, fat percentage, and BMI in those who trained. These results demonstrate that combined exercise can reduce the negative effects of diabetes on the bone (31).
2.2. Animal Studies
The effect of exercise on bone density in the animal model has been studied extensively (32-36). In these studies, the ovariectomy model was used in female animals (35, 36) or in male animals by injecting osteoporosis inducers (32, 33). Banparvari and Kaka examined the skeletal changes resulting from two increasingly loaded training programs on the bone characteristics of osteoporotic male rats (32). Thirty adult male Wistar rats with an average initial weight of 180 – 200 g were used in their study (32). Osteoporosis was induced by intraperitoneal injection of 20% ethanol solution (3 g/kg/day) for four consecutive days for three weeks (32). The training groups performed the training program five days a week for 12 weeks according to the resistance or endurance protocol (32). At the end of the intervention, the animals were sacrificed and the BMD of the femur and the fourth and fifth lumbar vertebrae (L4 + L5) were measured (32). The maximum tensile load of the left tibia and compression of the lumbar vertebra were measured by mechanical testing (32). The results showed that the endurance and resistance groups increased femoral BMD compared to the control group (32). L4 and L5 BMD of resistance and control groups were higher than the endurance group. The researchers concluded that resistance training compared to endurance training could induce more effective changes in mineral density and mechanical bone strength (32). Nazem and Salehikia investigated the effect of simultaneous aerobic and progressive resistance training on changes in BMD and mechanical strength of the femur in male rats with osteoporosis (33). Eight rats were isolated as healthy groups. Osteoporosis was then induced by peritoneal injection of 20% ethanol/saline solution (3 g/kg body weight) in the other rats (33). The injection was given on the first four days of the week, once a day for three consecutive weeks. Rats with osteoporosis were divided into four groups: Baseline, endurance training, combination training, and control (eight rats in each group) (33). The endurance training protocol included running on a treadmill with a zero-degree incline at a constant speed of 12 m/min for a maximum of 60 minutes per day. The combined training group performed two endurance and resistance training programs (four ascents from a 110 cm vertical ladder at 80°; weights attached to the tail; progressive overload starting at 50% of body weight in the first set and increasing 100% of body weight by the fourth set) (33). After completing 12 weeks of exercise (five days per week), the animals were sacrificed, and the bones of the left thigh removed were scanned to measure body mineral density and tested by a three-point bending test for maximum fracture strength and bone strength. The results demonstrated that the combined training group increased BMD, maximum fracture force, and stiffness in comparison to the control group. Endurance exercise caused an increase in maximum fracture force and stiffness but no change in BMD (33). Changes in BMD, maximum fracture force, and stiffness of the femur in the combined training group were significantly higher than in the endurance group. Based on this study, a combination of resistance and endurance training may synergistically increase the mechanical strength of male rat femurs versus endurance training alone (33). Hemmati Farsani et al. compared different endurance intensities on selected femoral biomechanical variables in elderly Wistar rats (34). Rats were divided into a moderate-intensity endurance training group (n = 8), high-intensity endurance training group (n = 8), and a control group (n = 8) (34). Moderate-intensity was 60 - 70%, and high-intensity was 80 - 110% of maximal speed. Training was performed five days per week for eight weeks (34). The training volume was equal in both groups, but the training intensity was different. Forty-eight hours after the last exercise session, the animals were sacrificed to remove femoral bones (34). Three-point bending tests determined changes in modulation, maximum fracture toughness and energy, and femoral strength were measured in male rats (34). No difference was observed between groups. They suggested that bone biomechanical changes after such exercises require longer periods of exercise, which should be studied in future research (34). Hojjati et al. investigated the effect of treadmill running on osteoporosis in ovariectomized rats (35). Thirty rats were randomly assigned into three groups: healthy control, ovariectomized control, and ovariectomized treadmill running. The rats’ body weight increased after 12 and 22 weeks post-ovariectomy (35). Ovariectomy reduced the thickness of cortical and trabecular bones and decreased femoral bone strength (35). The thickness of trabecular and cortical bone and femoral strength increased in the ovariectomized treadmill running group compared to ovariectomized control (35). These results suggest that weight-bearing exercise at moderate intensity has a protective effect against osteoporosis (35). Haji Sadeghi et al. investigated the effect of resistance training along with vitamin D and calcium intake in the premenopausal period in rats on BMD (36). The animals were randomly divided into control, placebo, vitamin D, calcium, resistance training, vitamin D + calcium, vitamin D + resistance training, calcium + resistance training, and vitamin D + calcium + resistance training. The control and placebo groups were fed a standard diet and sesame oil, respectively. Ovariectomy was performed after two months of resistance training, calcium, or vitamin D induction. Hematoxylin-eosin staining was performed to evaluate the diameter and number of bone cells. The results showed that resistance training increased lumbar BMD more than the other groups. The BMD in calcium and calcium + resistance training groups was higher than the control group (36). The number of osteoclast cells in the resistance training groups, vitamin D + resistance training, calcium + vitamin D, calcium + resistance training, and calcium + vitamin D + resistance training decreased compared to the control group. Based on these results, the authors concluded that regular resistance exercise, along with the consumption of vitamin D and calcium in the premenopausal period, increased bone density and minerals, which can delay osteoporosis during menopause (36).
3. Conclusions
A review of studies on the effect of exercise programs on bone density shows that regular PA with different patterns has a favorable effect on bone density in both human and animal models. Although several studies have not reported a significant effect on bone density, this can be interpreted as the maintenance of BMD in older females. This would mean the delay of osteoporosis occurrence and/or development as the evidence demonstrated a protective effect against BMD degradation with regular PA.