1. Background
Endocrine disrupting chemicals (EDC) are environmental compounds that arise from many different sources and act as hormone mimics to disrupt normal endocrine function. They lead to altered sexual development and reproduction in both sexes of human and wild life animals (1-4). One of these endocrine disrupting chemicals is Bisphenol A (BPA). BPA is used extensively in the industry and commerce to manufacture polycarbonate plastics. These plastics can be used in baby bottles, water storage tanks, or supply pipes (5). It is also used to make epoxy resins, electronics, medical equipments, and dental sealants (6, 7). The human population is frequently exposed to BPA through various sources. For instance, BPA can leach from the inner lining of tin cans and microwave containers into the food materials during heating (8) and from dental sealants into saliva (9) and from polycarbonate bottles into beverages due to repeated usage or contact with any acidic/alkaline contents (10).
This shows weak binding affinity to the estrogen receptors (ER) and scant estrogenic activity when measured using the yeast two-hybrid assay and antagonist for the androgen (11, 12), thyroid (13), and aryl hydrocarbon receptors (11). Reproductive physiology involves complex biological processes that can be disrupted when exposed to environmental contaminants (14). The male accessory sex glands of mammals include the prostate gland, the vesicular glands, the glands of the ampulla and the bulbourethral glands. These glands work by androgen, and play an important role in the reproductive function (15, 16).
The appearance of BPA in tissues and fluids of the human body has adverse effects on the male reproductive system. Significant damages to the acrosomes, the plasma membrane with reduced mitochondrial activity, and increased levels of defective spermatozoa may have compromised sperm functions, and may cause faster movement through epididymis BPA exposure, reducing the serum concentrations of testosterone, LH and FSH and increasing the concentration of estradiol (14). The accumulation of DNA damage in germ cells was induced by BPA exposure via oxidative stress (17). Exposing the adult male rats to BPA resulted in the decrease of testicular weight, daily sperm production and efficiency of spermatogenesis (18). Furthermore, prenatal exposure of pregnant rats to BPA causes breast cancer in adult female offsprings and hyperplasia of prostate in male rats, and this hyperplasia may result in greater risk of prostate cancer (19).
In humans, BPA also reduced sperm concentration, motility and morphology (17). High levels of BPA exposure, correlated with increased risk of mammary gland and prostate cancers have negative effects on the tissues of prostate and seminal vesicles glands.
2. Objectives
The aim of this study was to investigate the effects of BPA doses on the histological structure and ultrastructure of prostate and seminal vesicle glands.
3. Methods
3.1. Animals and Treatments
In this study, 40 four-week-old male Wistar rats (200 - 250 gr) were obtained from the experimental animal center of Urmia university. They were maintained in a controlled environment temperature and on a 12/12 light/dark cycle (lights on 7:00 am), with free access to rat chow and water. After two weeks of acclimatization period, the mice were randomly assigned into one control and three treatment groups, (n = 10), each receiving BPA at doses of 10-50-100 µg/kg, which was dissolved in ethanol and water for stock solution in 15 consecutive days or only in ethanol as a vehicle control during the same period. Bisphenol A was administrated intraperitoneally for 15 days.
3.2. Chemicals
Bisphenol A was obtained from Merck Co. Other chemicals such as ethanol 96% were used in this study as solvent.
3.3. Light Microscopic Analysis
The prostate and vesicular glands were dissected out, and tissue samples were fixed by immersion in 10% formalin. Then, the samples were dehydrated in graded ethanol, cleared in xylen, and impregnated and embedded in paraffin wax. Sections of 6 - 7 μ thick were obtained, utilizing Leitz microtome model 1512 and stained with hematoxylin/eosin (H & E) (20). All the slides were examined and photographed, using an Olympus BH-2 light microscope to be assessed by the histological features.
3.4. Electron Microscopic Analysis
For electron microscopy, prostate and vesicle seminal tissues were fixed for 10 - 30 minutes in acrolein and for two to six hours in a mixture of glutaraldehyde (2.5%) and paraformaldehyde (4%). They were then washed and stored. Furthermore, small blocks (1 mm) were cut from the large blocks and were used for ordinary frozen sections. The pieces were washed in graded series of ethanol (21). They were embedded, using pure resin and ultrathin sections and were stained with uranyl-acetate and lead citrate (22). The sections were examined with a LEO 906 transmission electron microscope (21).
4. Results
4.1. Light Microscopy
These observations revealed pathological changes in the prostate and seminal vesicle tissue, at doses of 50 and 100 µg/kg of BPA.
4.2. Prostate Analysis
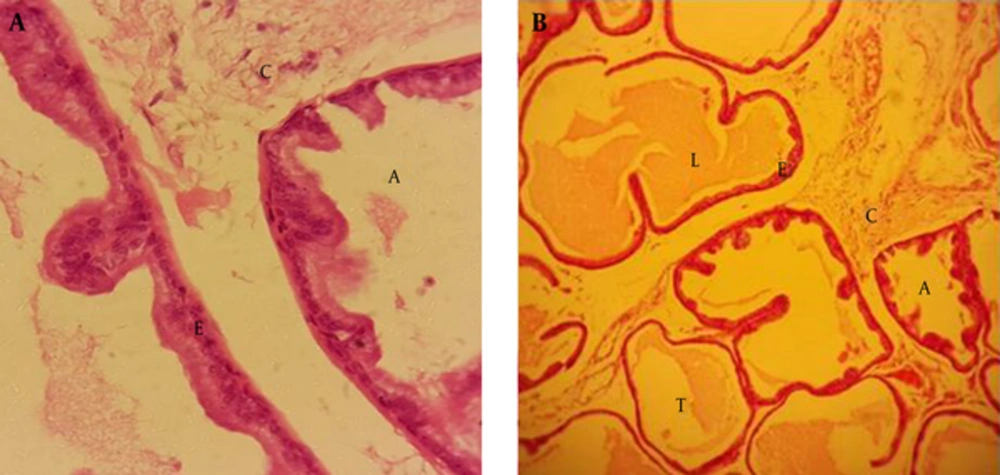
After examining the prostate gland, using a light microscope, we found that the structure of this gland consists of the matrix and parenchyma, formed by the tissue connected to nerves, blood vessels and smooth muscle fibers. Prostate epithelium is simple or pseudo-stratified columnar. It has alveolar and tubular secretory units, and a loose connective tissue, which can be seen between secretory units (Figure 1A and B).
After examining the rats treated with BPA at dose of 100 µg/kg, we found congestion in the blood vessels of the connective tissue, and this change could result in tissue destruction (Figure 2A and B).
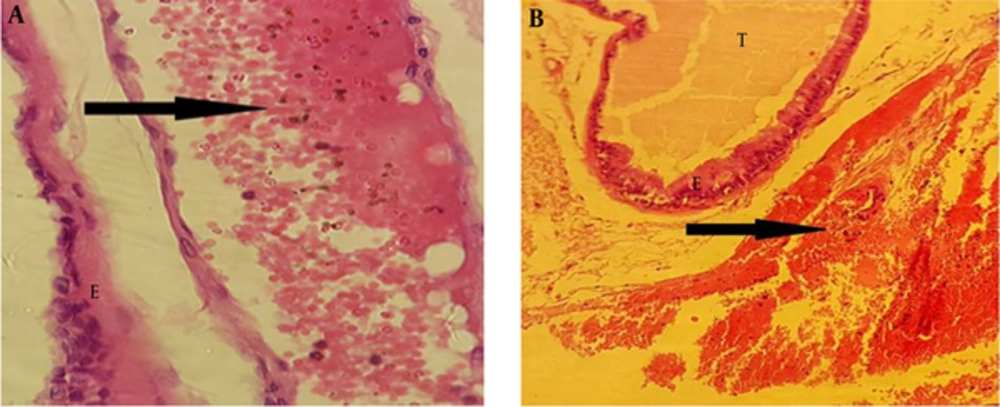
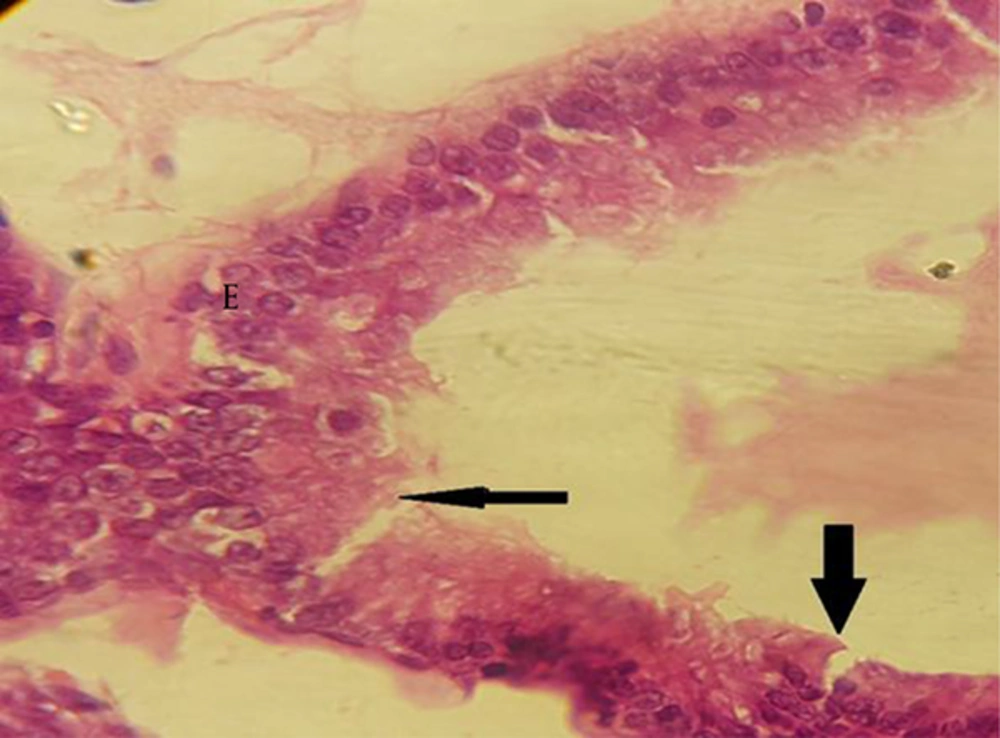
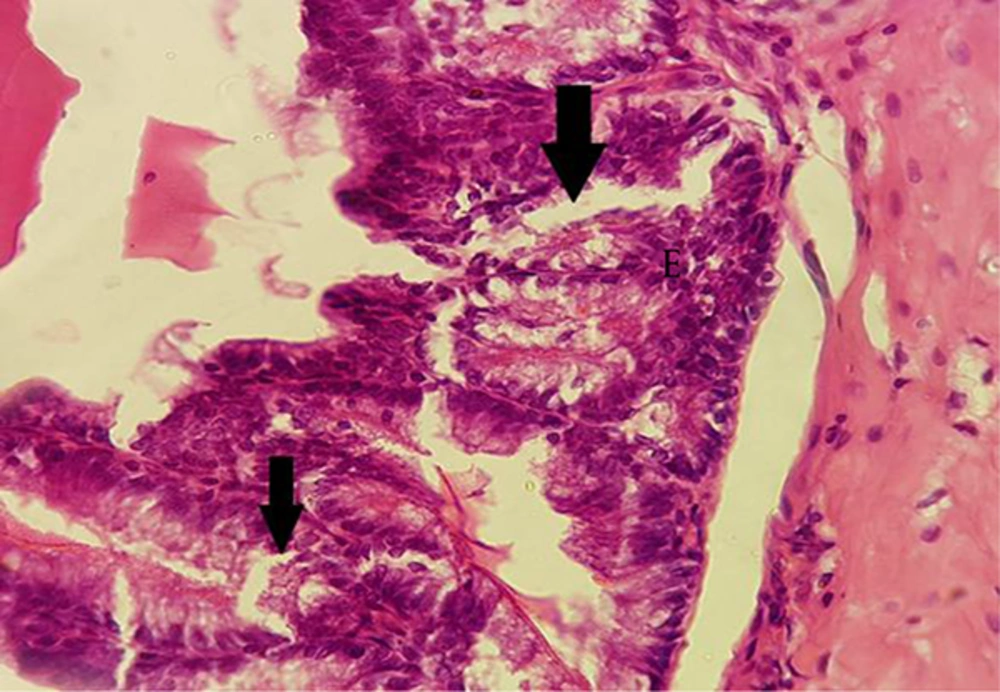
Administrated BPA at dose of 50 µg/kg showed rupture in epithelium of the secretory units compared to the control group. In addition, epithelium lost its integrity in the treated group (Figure 3).
4.3. Vesicle Seminal Analysis
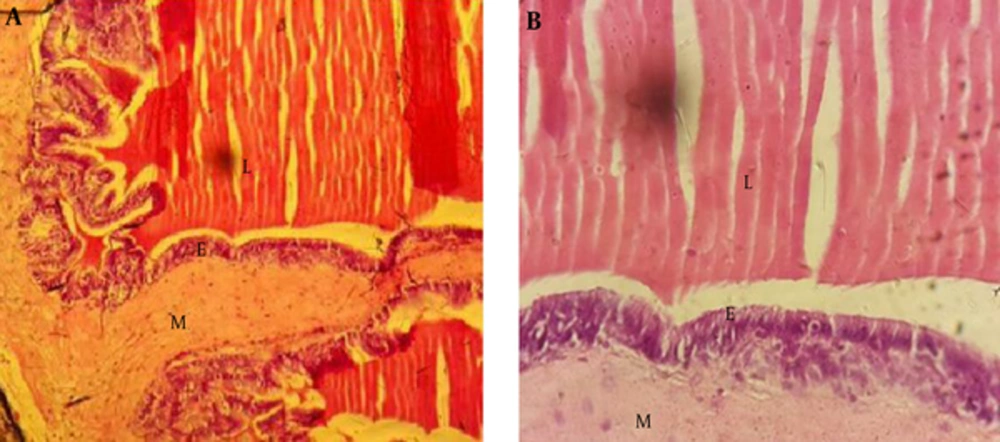
Examination of the vesicle seminal gland by a light microscope demonstrated that this gland includes mucous with many complex and thin folds covered by simple or pseudo-stratified columnar epithelium. Muscle layer was formed by smooth and circular muscles, and lumen that contained semen secretion (Figure 4A and B).
In the treated groups with BPA at dose of 100 µg/kg, rupture was observed in epithelium of the folds in comparison to the control group. Epithelium lost its integrity in the treated groups (Figure 5).
4.4. Electron Microscopy
4.4.1. Prostate Analysis
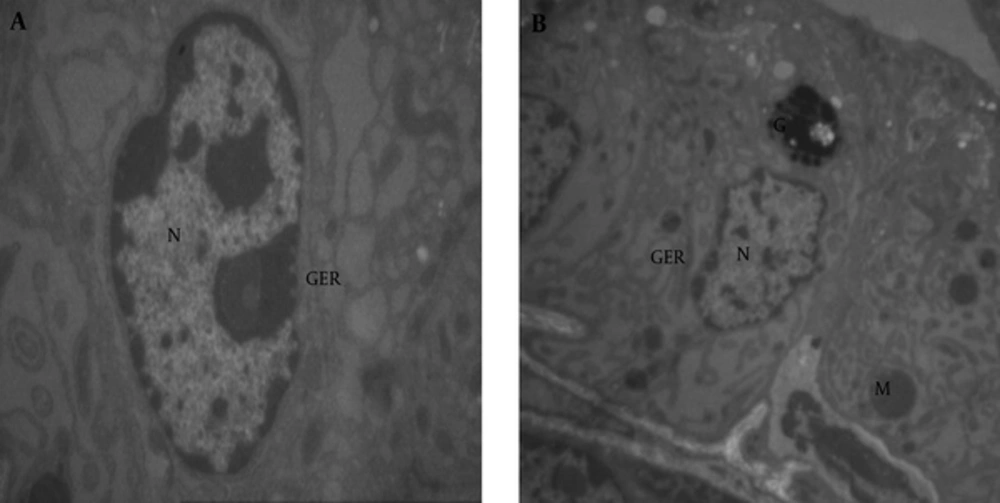
Using electron microscope, we could see cell organelles such as nuclei, nucleus, mitochondria and endoplasmic reticulum in the prostate tissue of the control group (Figure 6A and B).
In the treated rats with BPA at dose of 100 µg/kg, we observed the nuclei condensation and nucleus disappearance in cells of the prostate tissue in comparison with the control group; and the cells lost their activity (Figure 7).
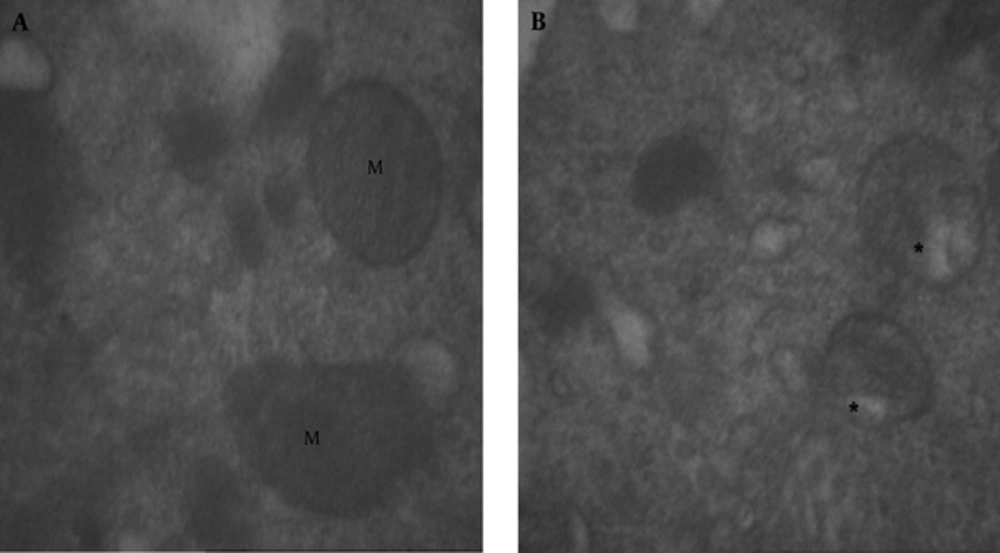
In the mitochondria of the treated group with BPA at dose of 100 µg/kg, we observed vacuolization (Figure 8B) in comparison with mitochondria of the control group (Figure 8A).
4.4.2. Seminal Vesicle Analysis
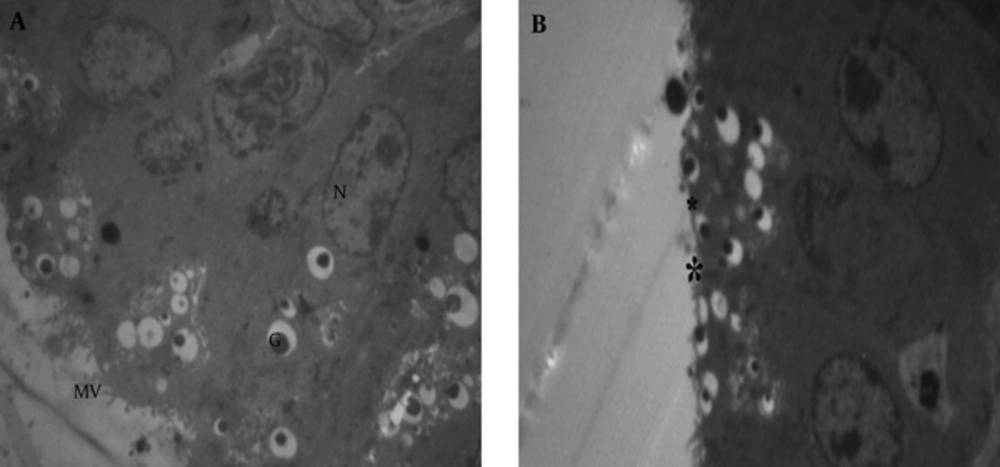
Normal tissues of the vesicle seminal in the control group included microvilli, nuclei, nucleus and other cellular organelles (Figure 9A). The microvilli of the treated group with BPA at dose of 100 µg/kg disappeared (Figure 9B).
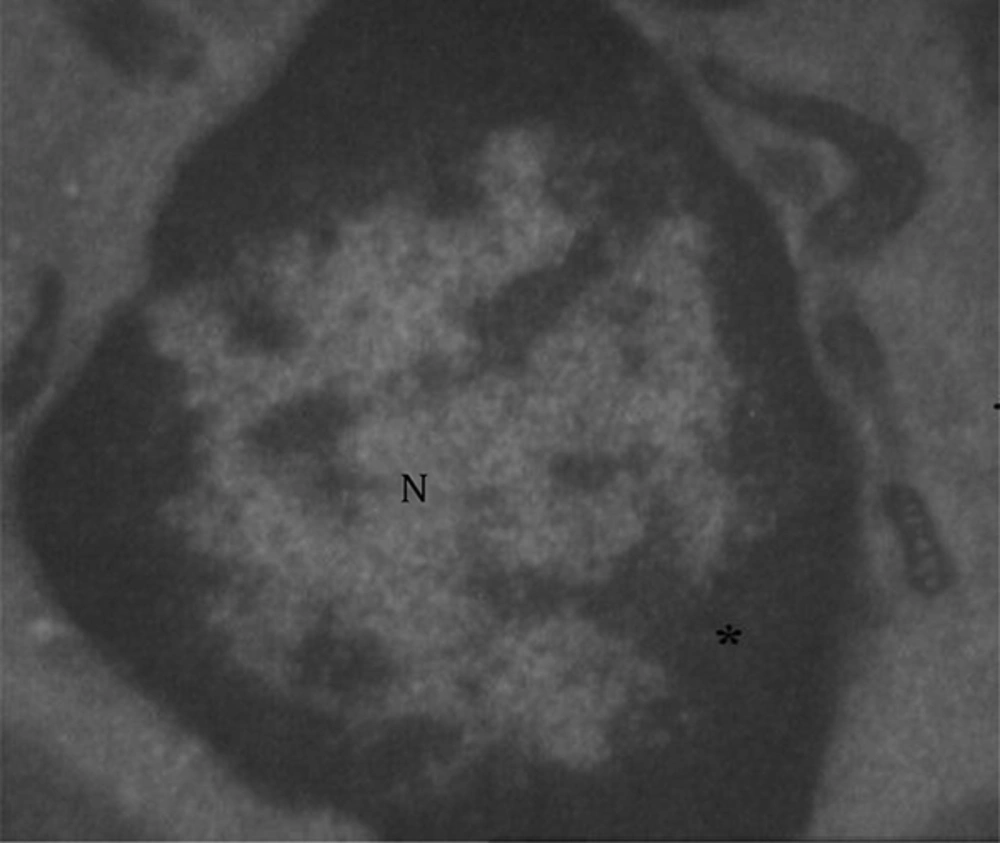
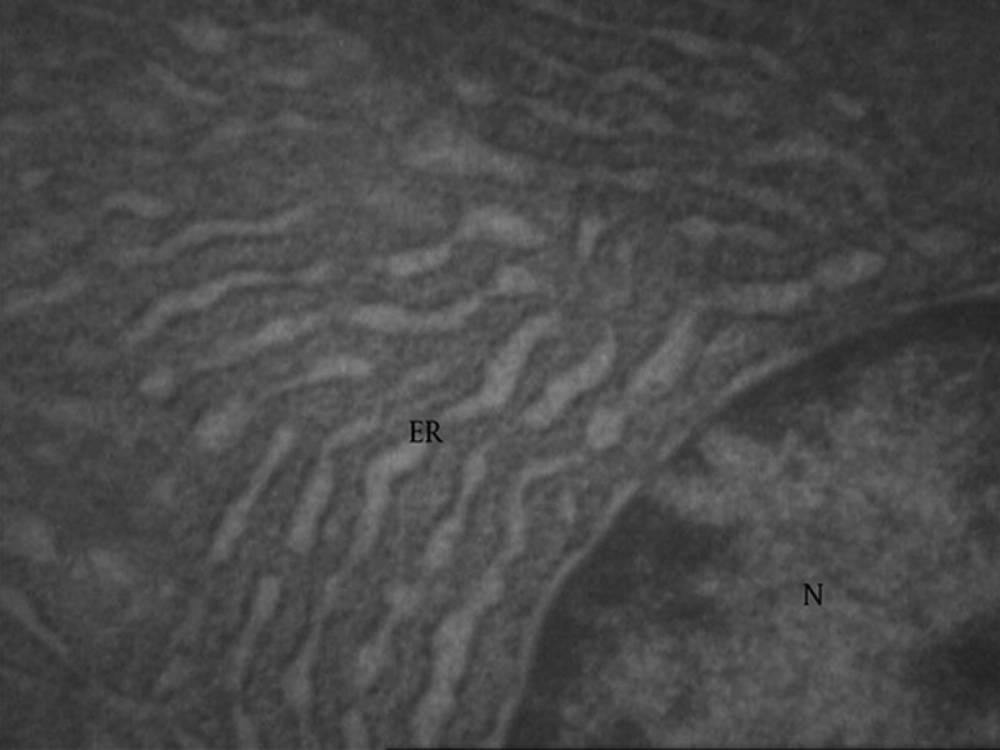
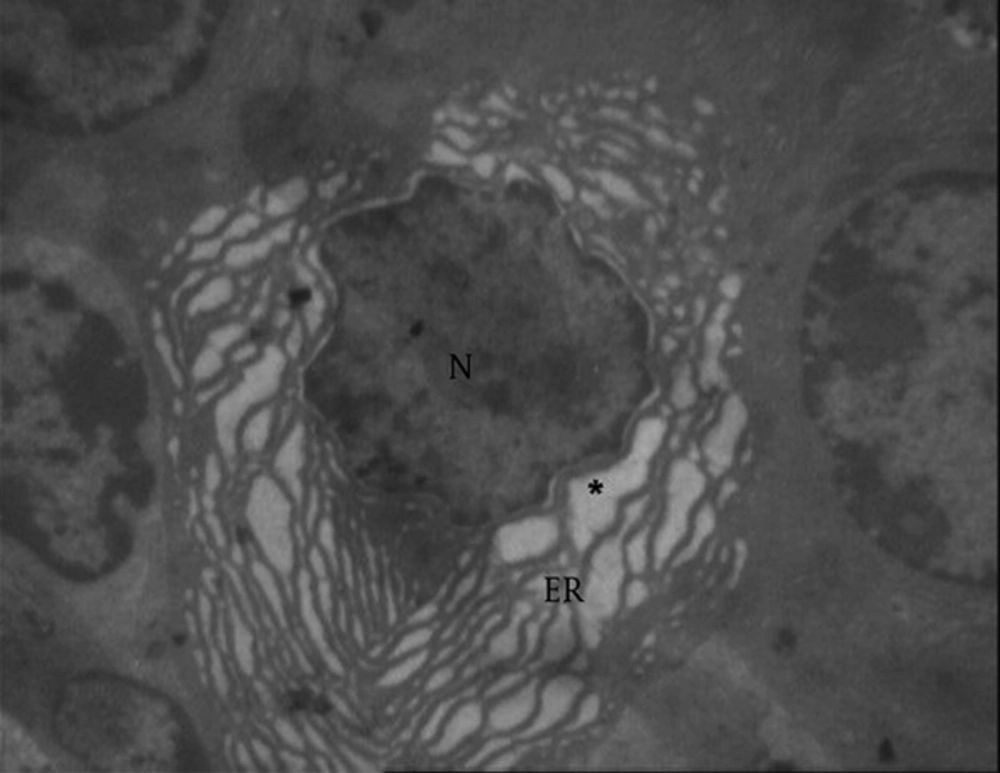
Normal endoplasmic reticulum in seminal vesicle (Figure 10) showed vacuolated reticulum (star and ER) endoplasmic around the nucleus (N) in the treated group, with 100 at 100 µg/kg dose (Figure 11).
5. Discussion
Bisphenol A (BPA) is a xenoestrogen, synthetized in large quantities to produce polymers and thermal paper, with a widespread use in manufacturing the products of everyday use. Data exist on BPA occurrence in food, water and indoor environments as well as its appearance in tissues and human body fluids. Bisphenol A is also an endocrine disruptor.
In this study, we administered Bisphenol A at doses of 10, 50, 100 μg/kg of body weight to the rats and detected pathological changes in both the structure and the ultrastructure study of prostate and seminal vesicle. These observations conducted on the prostate and seminal vesicle glands revealed congestion in the connective tissue, vacuolization in the epithelium of the secretory units and epithelium rupture at doses of 50 and 100 µg/kg of BPA. Ultra structure study of the prostate and seminal vesicle tissue demonstrated vacuolization of mitochondria, nuclei condensation and nucleus disappearance in the prostate tissue, vacuolization and dilation of ER and secretory glands. It also revealed the disappearance of microvilli and an increase in collagen fibers around the nuclei in the seminal vesicle in the treated group with BPA at dose of 100 πg/kg compared to the control group. This result revealed that BPA has destructive effects on the prostate and seminal vesicle glands. It also showed that BPA has adverse effects on the reproductive system in male rats.
BPA is reported to have both estrogenic and antiandrogenic effects (6, 23, 24). Several toxicological studies (25) pointed out that rodents exposed to BPA during the prenatal or perinatal period show a large variety of adverse reproductive outcomes, including decreased epididymal weight and daily sperm production (26, 27) and increased prostate weight (28), which is somewhat similar to our findings. With respect to the prepubertal or pubertal exposures, rodent studies have described a dramatic decrease in testosterone (T) levels (29, 30) and epididymal sperm counts (29) after BPA exposure. After being exposed to BPA at a dose of 25 or 100 ng/kg, adult male mice showed a significant reduction in testicular as well as epididymal sperm counts (31). Tohei et al. (2001) (32) reported that plasma concentrations of T were decreased and plasma concentrations of luteinizing hormone (LH) were increased in male adult rats, which were treated in different doses of BPA, compared to the rats in the control group.
This xenoestrogen is able to bind and activate both ERa and ERb (23) and is shown to suppress androgen production by rat Leydig cells in vivo and in vitro (33). However, the direct effects of BPA on androgen production, using developing mouse Leydig cells with different capacities, have not been investigated yet. Prepubertal Leydig cells produce androgens that play an important role in the initiation and maintenance of spermatogenesis as well as the regulation of multiple androgen-dependent physiological processes in the developing body (34).
The effect of low doses of Bisphenol A on male reproduction is controversial. Ema et al. (2001) (35) reported that administration of Bisphenol A at doses of 0.2/200 mg/Kg body weight per day did not cause significant changes in the reproductive parameters of the rats for two generations; however, our study showed that low doses of Bisphenol A caused reproductive toxicity.
Moreover, vitro and animal studies show that BPA exposure can increase the risk of mammary gland, brain, and prostate cancers (36). However, human studies linking BPA exposure to heightened cancer risk are scarce. Chronic long-term exposure of high-dose of estrogenic substances leads to squamous metaplasia of the prostate, which involves multi layering of the basal epithelial cells and expression of cytokeratin 10 (CK10) (37). Nevertheless, numerous findings suggest that BPA adversely affects the male reproductive system. Tohei et al. (32) showed that Bisphenol A inhibits testicular function in adult male rats. In mice, testicular hypotrophy and decreased daily sperm production were observed in the presence of BPA (31, 38). In humans, BPA also reduced sperm concentration, motility and morphology (39). BPA exposure may also induce apoptosis in rat germ cells in vivo (40) and in cultured rat Sertoli cells (41), and has the potential to redistribute several known Sertoli cell junctional proteins (42, 43). Subsequent studies also demonstrated that BPA is genotoxic. The accumulation of DNA damage in germ cells was induced by being exposed to BPA via oxidative stress (43).