1. Background
Insulin resistance (IR) as a risk factor for developing type 2 diabetes (T2D) performs a key role in hyperglycemia (1). IR is caused by the intersection of multiple factors, including diet and a sedentary lifestyle (2). It is also stated that high-sugar diets play an axial role in metabolic dysregulation, leading to diseases including IR (3). Improving IR remains an important area of research in treating T2D and metabolic syndrome due to the need for new insulin-sensitizing therapies (4).
Improving insulin sensitivity and glucose uptake by exercise training plays an axial role in reducing insulin resistance through various pathways (5). Emerging studies have shown that exercise training stimulates the secretion of proteins from various tissues into the bloodstream, contributing to the beneficial effects on metabolism (6).
Secreted proteins such as adipokines and myokines are closely associated with the development of IR and have drug development potential in treating such diseases (7). METRNL is a recently identified secreted adipomyokine that express in various tissues, including skeletal muscle and adipose tissue, and afterward enters the bloodstream (8, 9). METRNL increases energy expenditure and improves insulin sensitivity by inducing the expression of genes related to brown fats thermogenesis in mice (8, 9). However, further research mechanisms are needed to elucidate the mechanism of METRNL in insulin resistance (10).
Some studies have shown that METRNL secretion increases in muscle and white adipose tissue (WAT) through resistance exercise, increasing energy expenditure and improving insulin sensitivity (11, 12). Resistance training improves insulin sensitivity by focusing on increasing muscle mass and reducing body fat (5). However, the effect of RT on serum and WAT concentrations of METRNL has not been quite elucidated.
2. Objectives
Due to the effect of RT on target tissues for METRNL secretion, this study aimed to investigate the effects of eight weeks of RT on adipose tissue and serum levels of METRNL in rats fed a sugar solution.
3. Methods
3.1. Animals
Ethical principles and animal care were based on the Guidelines for the Care and Use of Laboratory Animals approved by the Ethics Committee of the University of Mazandaran (code: IR.UMZ.REC.1400.027). In this study, 32 Wistar male rats, 6 - 8 weeks old, were maintained at a standard animal lab under controlled light/dark (12/12 hours) and temperature (22 ± 2°C) conditions. After a week of acclimatization to their living conditions and how to exercise, the animals were randomly separated into 2 groups, including a normal diet and high sugar diet (30% (w/v) sucrose solution in water in water in a separate bottle) (13). After 4 weeks, each group was divided into control and resistance training groups.
3.2. Training Protocol
Rats in the training groups performed RT with the use of a ladder for three days per week and lasted 8 weeks. The initial training session with a load equivalent to 50% of the rat's body weight was repeated twice for the training group, 5 times for each round. During 2 weeks, the number of training sets gradually increased to 4 and 5 repetitions per set. Also, by observing the principle of overload in the third week and the following weeks, it was equal to 75, 100, 125, 150, 175, and 200% of the weight of rats, respectively, and the workload was constant in the final weeks (two weeks). The rats were weighed weekly to determine the appropriate weight for the training load. There was a 1-minute break between each repetition and a 2-minute break between each turn (14). Before and after each training session, the rats climbed the stairs 2–3 times without weights to warm up and cool down.
3.3. Sample Collection
After an 8-hour overnight fast, the animals were anesthetized with an intraperitoneal injection of a combination of ketamine (70 mg/kg) and xylazine (3 - 5 mg/kg) 72 hours after the last training session. Blood samples were taken from animal hearts and centrifuged at 3000 rpm for 15 minutes at 4°C to separate the serum. Epididymal white adipose tissue (eWAT) samples, after weighing, were homogenized in a buffer containing one mL of PBS buffer and 100 mg of adipose tissue powdered, followed by 10-min centrifugation at 4000 rpm; its supernatant was collected to evaluate Metrnl values. Serum and tissue samples were transferred to the freezer at minus 20°C for further investigation.
3.4. Biochemical Measurements
Insulin and METRNL levels were analyzed using ELISA kits (METRNL ZellBio Germany, insulin Mercodia Sweden). Serum glucose was measured by colorimetric assay using a glucose oxidase assay kit (Pars Azmoun Company, Iran). HOMA-IR index became calculated as follow: HOMA-IR; (insulin (mU/L) × glucose (mmol/L) /22.5)
3.5. Statistical Methods
Data normality was checked using Kolmogorov– Smirnov test. A two-way ANOVA was used to determine the main effects of the diet (normal vs. high sucrose), training status (control vs. trained), and their interactions. T-test was used when the two-way ANOVA test detected a statistical difference. To explore relationships between variables, Pearson's correlation coefficient was used. The statistical analyses were conducted using SPSS version 23, and statistical significance was set at P < 0.05.
4. Results
There were no significant differences in final body weight and changes in body weight between the groups (P > 0.05; see Table 1). The rats fed with a sugar solution had higher weights of epididymal and mesenteric fat (both absolute and relative) compared to rats on a normal diet (Table 1). Eight weeks of RT did not significantly affect the weights of fat pads.
| Variables | Normal Diet | Sugar Solution | Two-way ANOVA | ||||
|---|---|---|---|---|---|---|---|
| Control | RT | Control | RT | Sugar Diet | Training | Interaction | |
| Weight changes, g | 190 ± 27.3 | 193.2 ± 40.8 | 208.3 ± 50.2 | 213 ± 26.3 | 0.162 | 0.769 | 0.959 |
| Epididymal fat weight, g | 4.91 ± 1.09 | 4.77 ± 1.03 | 7.22 ± 2.87 | 7.53 ± 2.24 | 0.002 | 0.908 | 0.757 |
| Epididymal fat/body weight, % | 1.42 ± 0.27 | 1.36 ± 0.23 | 1.92 ± 0.60 | 2.05 ± 0.65 | 0.002 | 0.833 | 0.589 |
| Mesenteric fat weight, g | 2.54 ± 0.78 | 2.81 ± 0.90 | 5.26 ± 1.98 | 3.86 ± 1.09 | < 0.001 | 0.221 | 0.074 |
| Mesenteric fat/body weight, % | 0.73 ± 0.20 | 0.79 ± 0.21 | 1.42 ± 0.45 | 1.50 ± 0.30 | < 0.001 | 0.177 | 0.055 |
a The values are expressed as mean ± SD (n = 8 rats per group). Weight change = final weight - initial weight. fat to body weight ratio = (fat weight / final weight) × 100.
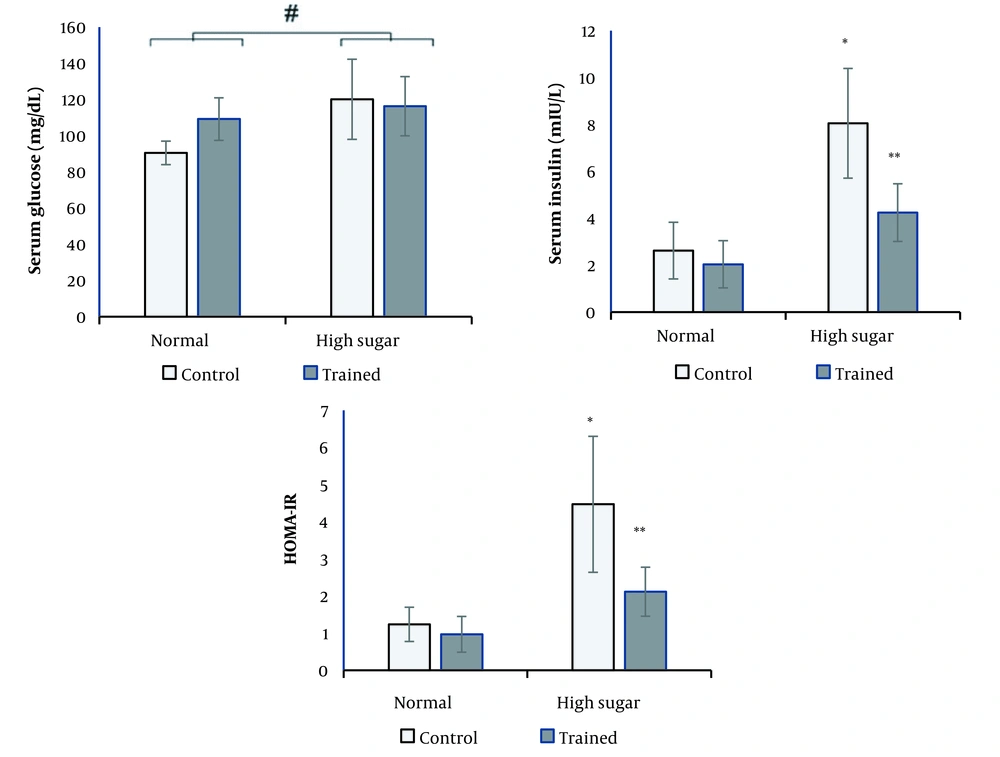
High sucrose diet significantly increased serum glucose (P = 0.004) levels (Figure 1). RT had no significant effect on serum glucose levels.
The interaction effects of two-way ANOVA were significant for insulin (P = 0.013) and HOMA-IR (P = 0.026). Therefore, we examined the effect of diet (by comparing normal control and high sucrose control groups) and training in different diet (normal or high sucrose) groups. The results of the t-test demonstrated that the insulin levels and HOMA-IR were significantly higher in the high sucrose control group compared with the normal control group (P < 0.05). Only in the high sucrose group RT significantly decreased serum insulin concentration and HOMA-IR compared with the high sucrose control group (P < 0.05; Figure 1).
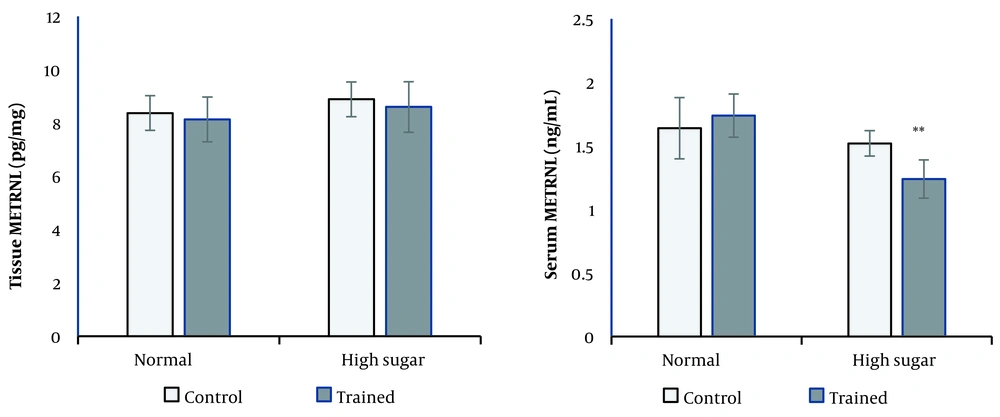
The interaction effects of two-way ANOVA was significant for serum METRNL concentration (P = 0.009). Only in the high sucrose group RT significantly decreased serum METRNL levels compared with the high sucrose control group (P = 0.002; Figure 2). Neither RT nor diet had a significant effect on adipose tissue METRNL levels (Figure 2).
We examined the simple correlation between mesenteric and epididymal fat weights and other metabolic or tissue and serum METRNL levels (Table 2). A positive correlation was observed between mesenteric fat weight and glucose levels (r = 0.611, P < 0.001), insulin (r = 0.621, P < 0.001), and HOMA-IR (r = 0.745, P < 0.001). Furthermore, positive correlations were found between epididymal fat mass and glucose levels (r = 0.522, P = 0.004), insulin (r = 0.560, P = 0.002), and HOMA-IR (r = 0.690, P < 0.001). There was no correlation between mesenteric and epididymal fat weight and tissue or serum METRNL levels.
| Variables | Mesenteric Fat Weight, g | Epididymal Fat Weight, g | ||
|---|---|---|---|---|
| r | P-Value | r | P-Value | |
| Glucose, mg/dL | 0.611 | < 0.001 | 0.522 | 0.004 |
| Insulin, mIU/L | 0.621 | < 0.001 | 0.560 | 0.002 |
| HOMA-IR | 0.745 | < 0.001 | 0.690 | < 0.001 |
| Tissue METRNL, pg/mg | 0.132 | 0.50 | 0.136 | 0.499 |
| Serum METRNL, ng/mL | -0.328 | 0.95 | -0.305 | 0.129 |
Abbreviations: HOMA-IR, homeostasis model assessment of insulin resistance.
5. Discussion
The main results of this study were that the animals that consumed a continuous 30% sugar solution showed an increase in mesenteric and epididymal fat weights, as well as elevated levels of serum glucose, insulin, and IR index. At the same time, there was a decrease in the serum levels of METRNL. On the other hand, eight weeks of RT improved sugar solution-induced metabolic disturbances such as serum insulin levels, IR index, and lower serum METRNL levels.
The findings of this study revealed that serum levels of METRNL partially decreased due to continuous consumption of sugar solution. Since serum insulin levels and IR index increased after consumption of sugar solution in the present study, accumulation of adipogenic compounds can also be a factor in decreasing serum METRNL. A study showed that elevated METRNL serum levels may increase the risk of T2D due to developmental IR (10). The results of the present study are in line with the results of Loffler et al. (15), Lee et al. (16), and El-Ashmawy et al. (17). They concluded that accumulation of adipogenic compounds, such as insulin, inhibits the expression of METRNL.
It has been shown that 8 weeks of RT did not lead to a significant change in serum METRNL levels. The results of Rao et al. (11) and Eaton et al.'s (12) showed that muscle damage caused by exercise is an effective factor in increasing the secretion of METRNL because the hormone METRNL invokes immune cells involved in tissue repair (18, 19). METRNL secretion is primarily performed by macrophages as a response to local injury (18). Based on the findings, METRNL responds to acute metabolic challenges such as a single bout of resistance exercise and acute cold pressure; further research is needed on the compatibility of this hormone with regular RT.
The results of the present study demonstrated that a combination of sugar-soluble feeding and RT led to a further decrease in serum METRNL levels. While most studies suggest that RT increases METRNL levels in the body, there are several possible mechanisms that could explain the synergistic effect of combining RT and sugar-soluble feeding in decreasing serum METRNL levels. Elevated serum METRNL levels have been associated with chronic inflammation that may have a potential therapeutic role in inflammation (20). In our study, the maximum carrying capacity increased after the second week of RT until the end of the program. Studies show that RT reduces inflammatory cytokines by increasing strength and muscle mass (21). RT and sugar-soluble feeding can affect hormonal responses, which play a role in insulin sensitivity (2, 22). RT has been shown to decrease inflammation markers (23), while consuming sugar-soluble foods after exercise may further reduce inflammation by increasing the availability of nutrients for muscle repair and recovery (24). The synergistic effect of combining RT and sugar-soluble feeding in reducing serum METRNL levels may involve multiple mechanisms that act together to improve insulin sensitivity.
There was no change in the METRNL levels of epididymal white adipose tissue (eWAT) in our study, in line with the results seen in the study of Rao et al. (11). A study of thermogenic stimulus regulation showed that acute cold pressure could significantly increase the expression of the METRNL gene in eWAT fat, while RT may not affect it (11).
The findings of the present study showed that fat pad weights increased due to continuous consumption of sugar solution. It seems that visceral white adipose tissue depots such as mesenteric uptake the Glucose for use in triacylglycerides (TAG) production (25, 26). It is well established, along with obesity, that the WAT presents an increase in inflammatory markers and infiltration of immune cells (27). The results of the present study are in line with some studies. They concluded that IR has a close correlation with visceral fat obesity (28).
5.1. Conclusions
RT appears to reduce the serum METRNL levels of sugar-fed rats. It can be attributed to the decreased adipose tissue inflammation, which in turn can be improved IR.