1. Background
Obesity causes several metabolic disorders in skeletal muscles (1, 2), including insulin resistance resulting from disturbances in glucose uptake and metabolism (3). Obesity increases the absorption and storage of fatty acids and decreases lipolysis in the skeletal muscle, thereby disrupting the turnover of fats (4). On the other hand, the oxidation of fatty acids is slowed down in skeletal muscles due to obesity (5), possibly because of an increase in the activity of lipogenic enzymes and a decrease in the oxidation capacity of mitochondria (6). For this reason, in obese individuals, fatty acids tend to be stored in skeletal muscles instead of being oxidized (7). One of the important genes involved in lipid metabolism is sterol regulatory element binding protein (SREBP) (8). As a group of transcription factors attached to the cell membrane, SREBPs regulate the expression of the genes controlling the production and absorption of cholesterol, fatty acids, triglycerides, and phospholipids (9). Evidence shows that SREBPs are expressed in tissues with high lipogenic capacity, such as hepatocytes and adipocytes. In vitro and in vivo studies have shown that this gene is also expressed in a significant portion of skeletal muscles (10). SREBPs have three isoforms: SREBP-1a, SREBP-1c, and SREBP-2. Among these, SREBP-1c, as a transcription factor, regulates the expression of lipogenic genes, such as fatty acid synthase (FAS) and malonyl-CoA and acetyl-CoA carboxylase (ACC) (11). The expression of SREBP-1 in the skeletal muscle tissue is partly regulated by insulin, and its expression is reduced during insulin deficiency (12).
It has been reported that SREBP-1c gene expression is increased due to aerobic training (11), while resistance training seems to have no significant effect on the expression of this gene (13). Nadeau et al. showed that both exercise and caloric restriction increase the expression of the SREBP-1c gene in skeletal muscles, which was consistent with elevated intramuscular triglyceride and insulin sensitivity (14)
2. Objectives
Since the effect of aerobic exercise on the expression of the SREBP-1c gene is not well known in obese individuals, this study was conducted to investigate the effect of aerobic exercise on SREBP-1c gene expression in the skeletal muscle tissue of obese rats.
3. Methods
3.1. Animals
In this experimental study, 18 female Wistar rats in the weight range of 180 to 200 grams were selected. After one week of adaptation to and familiarization with the research environment, the animals were randomly divided into three groups: (1) control animals fed with a normal diet; (2) control animals fed with a high-fat diet; and (3) aerobic exercise + high-fat diet. All animals were kept under standard laboratory conditions in transparent polycarbonate cages at autoclavable temperature (20 - 22 ºC), relative humidity of 55%, 12-hour dark/light cycles, and free access to sufficient water and food provided by Behparvar Co. (Iran). The rats were kept and studied according to the instructions for working with laboratory animals approved by the Ministry of Health and Medical Education of the Islamic Republic of Iran.
3.2. High-fat Diet
A high-fat diet containing 40% fat (20% soybean oil and 20% animal fat), 13% protein, and 47% carbohydrates was used to induce obesity. The rats in the experimental group were fed with high-fat diet for 6 weeks, followed by aerobic exercise for 6 additional weeks. Normal or high-fat diets in the form of pellets were fed to control animals without aerobic exercise.
3.3. Aerobic Exercise Protocol
The exercise program used in this study lasted six weeks, with five sessions per week of moderate-intensity running on a treadmill. The intensity of training reached 50% VO2max in the first week and 65% VO2max in the last week. In order to acclimatize the rats, before starting the main training program, they performed training for one week at a speed of 9 m/min for 20 minutes. The duration of training was 15 minutes in the first week, and 5 minutes was added to this time every week. Training intensity was 14 m/min on the first day, and the running speed increased 3 m/min every week. Each exercise session started with a 5-minute warming up and concluded with a 5-minute cooling down, both at a speed of 10 m/min.
3.4. Animal Sacrifice, Quadriceps Muscle Removal, and SREBP-1c Gene Expression Assessment
Forty-eight hours after the last session of aerobic exercise and after 12 hours of fasting, the rats were anesthetized by the intraperitoneal injection of ketamine and xylazine and sacrificed by drawing blood from the left ventricle. The quadriceps muscle tissue was removed and placed in a 2 mL microtube. The microtube was transferred into a nitrogen tank and kept in a -80 ºC freezer until analysis. Real-time PCR was used to investigate SREBP-1c gene expression in the quadriceps muscle. First, primers were designed for the target gene, and then total RNA was extracted from the muscle tissue and converted into cDNA. After this step, the cDNA was amplified by PCR, and SREBP-1c gene expression was quantified. The primers used in this study have been listed in Table 1.
| Gene Name | Forward | Reverse |
|---|---|---|
| SREBP-1c | 5′-GGA GCCATGGATTGCACATT-3′ | 5′-AGGAAGGCTTCCAGAGAGGA-3′ |
| GAPDH | AAGTTCAACGGCACAGTCAAGG | CATACTCAGCACCAGCATCACC |
Abbreviations: SREBP-1c, sterol regulatory element binding proteins-1c; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
3.5. Statistical Procedures
The Shapiro-Wilk test was used to determine the normality of data distribution. The homogeneity of variances was also assessed using Levene’s test. The expression of the SREBP-1c gene was compared between the studied groups using the one-way analysis of variance and Tukey’s post-hoc test. A P value of < 0.05 was considered as the statistical significance level. All calculations were performed using SPSS version 25 software.
4. Results
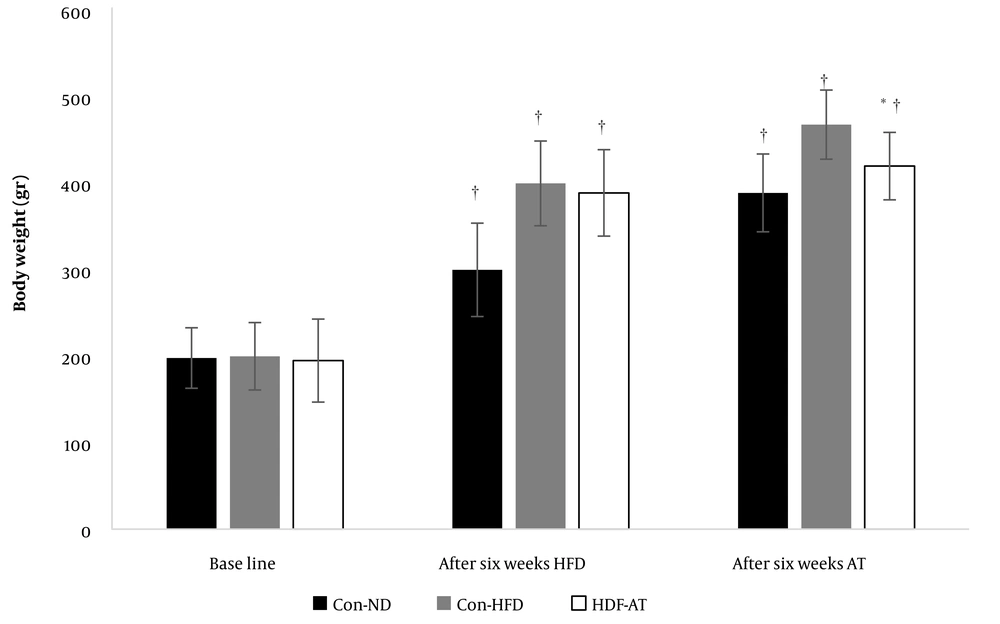
The Shapiro-Wilk test and Levene’s test affirmed the normality of data distribution and homogeneity of variances, respectively. The results showed that feeding with a high-fat diet significantly increased the weight of the animals (P = 0.001), and aerobic exercise resulted in significant weight loss (P = 0.001, Figure 1).
Weight changes in the normal diet and high-fat diet control groups and the high-fat diet plus aerobic exercise group († indicates a significant difference compared to the baseline and after six weeks of feeding with HFD; * shows a significant difference compared to the high-fat diet control group. Data are based on mean and standard deviation).
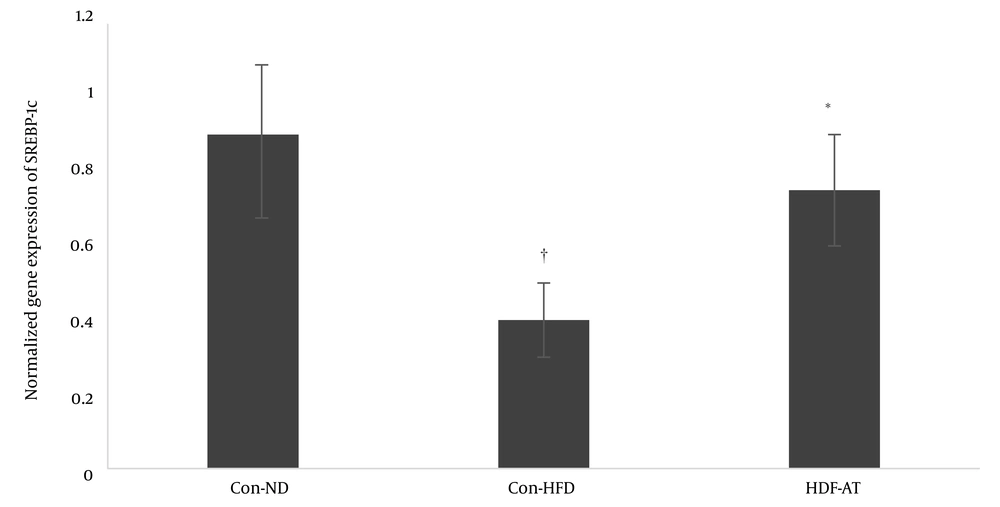
Feeding with a high-fat diet decreased the expression of the SREBP-1c gene significantly (P = 0.001), while aerobic exercise increased the expression of this gene (Figure 2).
5. Discussion
The present study showed that feeding with a high-fat diet significantly decreased the expression of the SREBP-1c gene. It is well-known that consuming a high-fat diet and, subsequently, obesity disturb lipid metabolism in the skeletal muscle tissue (4). One of the possible mechanisms for the reduction of SREBP-1c gene expression in the skeletal muscle tissue can be the disruption of the insulin signaling pathway in the skeletal muscle. It has been reported that SREBP-1c gene expression in the skeletal muscle is regulated by the insulin signaling pathway, including the PI3k/AKt/mTOR route (15, 16). Disturbance in the insulin signaling pathway causes a decrease in SREBP-1c gene expression (17). In the present study, insulin sensitivity was not assessed, but according to the available literature, significant weight gain due to consuming high-fat diets is associated with insulin resistance in the muscle tissue (18, 19). Confirming the effect of insulin on the expression of the SREBP-1c gene, it has been reported that a decrease in insulin in diabetic rats treated with streptozotocin significantly lowered the expression of this gene in the liver tissue (20). On the other hand, in diabetic rats treated with streptozotocin, the expression of the SREBP-1c gene in the skeletal muscle was shown to decline significantly (21). These findings show that in the skeletal muscle, the expression of SREBP-1c is sensitive to insulin, where this transcription factor can act as a regulator to modulate the effects of the hormone on the genes involved in lipogenesis and, therefore, has a central role in long-term sensitivity to muscle insulin. Based on this, the negative effects of consuming a high-fat diet on lipid metabolism in the skeletal muscle can be justified by SREBP-1c downregulation at the gene and protein levels.
Another finding of this study showed that aerobic training caused weight loss accompanied by increased SREBP-1c gene expression in the quadriceps muscles of the rats fed with the high-fat diet. It was reported that fifty minutes of aerobic exercise, five sessions a week for eight weeks, increased the expression of the SREBP-1c gene in the liver tissue (22). Similar studies in the skeletal muscle tissue also showed that aerobic exercise increased the expression of the SREBP-1c gene (11, 14, 23). Insulin sensitivity is one of the molecular mechanisms that can justify the increase in SREBP-1c gene expression in the skeletal muscle tissue. As mentioned, insulin increases the expression of the SREBP-1c gene in the skeletal muscle. It is well-established that aerobic exercise triggers weight loss and reduces insulin resistance in peripheral tissues (24). It seems that aerobic exercise in the present study facilitated weight loss, increased insulin sensitivity, and, thus, increased the expression of the SREBP-1c gene. Another mechanism that can explain exercise-induced SREBP-1c upregulation in the skeletal muscle is the MAP kinase signaling pathway. It has been reported that insulin increases the expression of the SREBP-1c gene in the skeletal muscle through the MAP kinase pathway (21, 25). On the other hand, several pieces of evidence show that regular physical activities enhance the MAP kinase signaling pathway in the skeletal muscle (26, 27). Also, SREBP-1c gene upregulation in the present study may be attributed to the cholesterol received from the high-fat diet. Evidence shows that increased cholesterol intake decreases SREBP-1c gene expression, so a decline in cholesterol can boost the activity of this enzyme (28, 29). In the present study, consuming a high-fat diet decreased the expression of the SREBP-1c gene, while aerobic exercise upregulated this gene. Since regular physical activity reduces cholesterol levels by various mechanisms, the cholesterol-lowering effect of aerobic exercise can justify the increase in SREBP-1c gene expression in this study.
5.1. Conclusions
In conclusion, the findings obtained from this study showed that feeding rats with a high-fat diet decreased the expression of the SREBP-1c gene in the quadriceps muscle, while aerobic training increased the expression of this gene. Accordingly, it can be attested that aerobic training is able to alleviate lipid metabolic disturbances in muscles by increasing the expression of the SREBP-1c gene. Therefore, it is recommended to use aerobic exercise to prevent metabolic disorders caused by high-fat diets in the skeletal muscle tissue.