1. Background
Heart failure (HF) refers to the inability of the cardiorespiratory system to respond to tissue demands, manifested by symptoms such as fatigue, dyspnea upon raising exercises, or even dyspnea at rest (1). The inability to do sports without inconvenience can be one of the first symptoms of HF, which is the leading cause of visiting healthcare centers. In other words, intolerance of sportive activities is inseparably associated with HF diagnosis. Ventricular performance indices are expected to be closely associated with sports activity capacity (1). The heart is vital in homeostasis and plays an inevitable role in life. As such, cardiac rehabilitation of HF patients is highly prioritized to improve their performance and alleviate their symptoms (2). Cardiac rehabilitation is a complex intervention involving sports exercises, enhancement of physical activities, health education, cardiovascular risk management, and psychological support. These issues can be personalized depending on patients' needs (3). Findings of randomized and controlled trials support the ability of cardiac rehabilitation as an effective and cost-effective clinical intervention for HF patients with decreased ejection fraction (4).
Previous studies have shown the association of HF with declined injection fraction (5), which may consequently decrease cardiac output activities and affect oxygen consumption. Heart failure has been shown to correlate with declined cardiac output, reduced sports activities, and sport-related dyspnea, all of which lower the performance capacity of respiratory and peripheral muscles (6-8). In addition to HF patients' heart problems, their skeletal muscles, especially respiratory muscles, are weakened. Based on the European Society of Cardiology (ESC) recommendation, 30 - 50% of HF patients suffer from muscular sarcopenia and disorders both in respiratory and nonrespiratory muscles (2). More recent studies have shown that the addition of respiratory rehabilitation can further improve symptoms, especially in patients with declined power and strength of respiratory muscles. Dall'Ago et al. (9) reported that respiratory muscle exercises can decrease the respiratory works and metabolic cost of respiration, hence declining dyspnea and increasing exercise tolerance (2, 9). Weak respiratory muscles with lower cardiac output have been observed in one-third to half of the patients (10).
Only three reviews and meta-analyses have explored the effect of respiratory rehabilitation exercises on maximum respiratory power and quality of life. In these studies, the quality of life and respiration power showed a remarkable improvement, while the peak exercise capacity and the duration of sports activities exhibited no significant enhancement (11), highlighting the need for further studies. Moreover, cardiac performance-related factors such as injection fraction and improvement of sports activities with these rehabilitation programs may require further investigations. Besides, the fact that respiratory muscle exercises along with rehabilitation can improve the performance and physiological conditions of HF patients still has some ambiguities. In recent years, the prevalence of diseases such as viral ones has prohibited the attendance of patients with underlying diseases in public places, and thus, many people with similar diseases have remained at home.
2. Objectives
The present study aimed to investigate the influence of eight weeks of home cardiorespiratory rehabilitation exercises on the cardiac performance of HF patients aged 40 - 60 years.
3. Methods
3.1. Ethical Considerations
All the stages of the present research were conducted according to ethical codes approved by the Ethics Committee of Tehran University of Medical Sciences (IR.TUMS.NI.REC.1399.042).
3.2. Participants
This was a quasi-empirical applied study. The statistical population includes HF patients visiting Baharloo and Shariati hospitals from 2020 to 2021. A cardiologist first examined all the patients, and their demographic information, clinical symptoms, cardiovascular risk factors, and medications were listed. The participants signed an informed written consent form. Forty-five patients were included and divided into 3 groups. The thirty patients willing to participate and pursue rehabilitation programs were randomly classified into 2 groups. One group did home aerobic exercises (n = 15; 13 cases remained at the end of the research); the other group performed respiratory exercises in addition to aerobic exercises (n = 15). The third group was the controls, who were unwilling to pursue the rehabilitation program (n = 15). The patients were first briefed on the aim of the study. All three groups received common medications. The inclusion criteria were HF patients aged 40 - 60 years with cardiac performance < 40% and ejection fraction < 40%, cardiac performance class 2 and 3, and no changes in the medication regime in the three months before the intervention. The exclusion criteria had acute coronary syndrome; uncontrolled cardiac arrhythmia; uncontrolled blood pressure (BP); advanced and untreated heart block; symptomatic myocarditis, pericarditis, and aorta stenosis; severe hypertrophic obstructive cardiomyopathy; acute and severe systemic disease; internal cardiac thrombosis; exercise intolerance for sports activities with severity below 3 metabolic equivalents (METs); resting dyspnea in the last three to five days; significant ischemia during low-intensity sports activities; uncontrolled diabetes; recent emboli; atrium thrombophlebitis fibrillation or a new atrium flatter; weight gain of more than 1.8 kg in the last 1 to 3 days; reduced BP upon sports activities; resting heart rate higher than 100; and Class IV NYHA (New York Heart Association) function.
3.3. Exercise Protocol
The rate of perceived exertion (RPE) expresses the extent of work or activity pressure. The exercise involved low-to-moderate sports with a hardness degree of 8 - 13. The Burg measure in the range of 6 - 20 was used, in which 6 implies no effort while 20 means maximum pressure on the person. The mild physical activities were in the range of 6 - 10, whereas the moderate and severe activities were in the ranges of 11 - 13 and 14 - 20, respectively (12). Aerobic exercise sessions included walking. Each session was recommended to take 30 - 60 min, including 5 m of warm-up and 5 m of cool-down.
The rehabilitation exercises were performed three times a week for 8 weeks. The patients started with two chairs placed 3 m apart. They did five exercises in two 15-minute stages. After 3 weeks, the patients visited the physician for examination. When there was no problem, the same exercises were repeated for a longer time (50 min), and then the patients visited the physician after 6 weeks. At this stage, 2 additional exercises were performed, and the patients did 7 exercises for 1 hour in the remaining two weeks. At the end of the eighth week, they visited the Sepehr Cardiology Center of Baharloo hospital (Table 1). The exercises were trained using videos (13-15).
| Steps | Details |
|---|---|
| Before rehabilitation | History, CPET, echocardiography, and measurement of the ability of respiratory muscles (Pimax) |
| 1st - 3rd weeks | 8 - 9 based on the Burg measure |
| 5 exercises with a chair | |
| Two 15-minute steps in the morning and afternoon | |
| 5 - 10 min of warm-up and cool-down | |
| 4th - 6th weeks | 10 - 11 based on the Burg measure |
| 5 exercises with a chair | |
| Two 15-minute steps in the morning and afternoon | |
| 5 - 10 min of warm-up and cool-down | |
| 7th - 8th weeks | 12 - 13 based on the Burg measure |
| 5 exercises with a chair | |
| Two 30-minutes steps in the morning and afternoon | |
| 5 - 10 min of warm-up and cool-down | |
| Pulmonary exercise protocol | The pulmonary muscle exercises were trained in the first session, and the patients were asked to do these exercises at home. A domestic model of the respiratory rehabilitation device was also delivered to them. The exercises took 15 - 30 min with 10 - 30% Pimax intensity. |
| At the end of rehabilitation | CPET, echocardiography, and measurement of the ability of respiratory muscles (Pimax) |
Abbreviations: CPET, cardiopulmonary exercise test; Pimax, maximal inspiratory pressure.
The respiratory exercise group received pulmonary in addition to aerobic exercises. The intervention was as follows: The respiratory muscle exercises were trained in the first session, and the patients were asked to do these exercises at home. A domestic model of the respiratory rehabilitation device (Powerbreathe Classic Health) was also delivered to the patients. The exercises took 15 - 30 min with 10 - 30% maximal inspiratory pressure (Pimax) intensity. They visited one week later for evaluation or troubleshooting if needed (Table 1). Heart rate, systolic and diastolic BP, ejection fraction, ventilatory threshold, and maximal oxygen consumption (VO2 max) were measured in the first session and 24 hours after the last session (13-15).
3.4. Cardiopulmonary Exercise Testing
All cardiopulmonary exercise testing (CPET) exercises were performed under the supervision of a cardiologist. Before the test, the patients were evaluated in terms of resting BP, heart rate, and echocardiography results, including the ejection fraction of the left ventricle. Patients with continuous chest pain at rest, BP drop, significant bradycardia/tachycardia, and ST segment deviations higher than 0.05 mV in the electrocardiography at rest were prohibited from doing the test. All the patients underwent CPET based on the modified Bruce protocol using two treadmills (Sport Test System, GE HealthCare, Chicago, IL, USA). The test took 16 min and 40 s, with a minimum recovery time of 5 min. Speed and degree were increased every 20 seconds. All the patients were encouraged to conduct a submaximal test with limited signs. The termination criteria included the patients' request, ventricular arrhythmia (ventricular tachycardia or fibrillation), or ST drop. The VO2 peak was defined as the maximum VO2 during the exercise (16).
3.5. Evaluation of Respiratory Muscles
The strength of the inhalation muscles can be assessed by Pimax. Upon the participant's request, this index is measured at the mouth level at the maximum remaining volume for at least 1 s. In the force-length equation, the higher the diaphragm's position (i.e., the longer the resting diaphragm's length or, the smaller the lung's volume), the higher the Pimax. This measurement is independent of the respiration flow and has high repeatability. Pimax < 70% of the predicted value indicates a weakness in respiratory muscles in HF patients. The strength of the inhalation muscles refers to their ability to maintain a definite respiratory pressure over time. This parameter can be measured by different methods. One way is to ask the subject to maintain Pimax over time to determine maximal stable inspiratory pressure (17). For this purpose, a spirometry device was used in this research.
3.6. Statistical Methods
The pretest and posttest scores were measured in the three groups. An analysis of covariance was employed to control the difference between the subjects and the effect of the covariate. Data analysis was performed in SPSS v. 23 (IBM, Chicago, IL, USA) at the significance level of P ≤ 0.05.
4. Results
Table 2 lists the subjects' specifications. The mean values of all the groups were similar, with no significant difference (P > 0.05).
| Groups | N | Age (y) | Height (cm) | Pre-weight (kg) | Post-weight (kg) | Pre-BMI (kg/m2) | Post-BMI (kg/m2) | |
|---|---|---|---|---|---|---|---|---|
| 1 | C | 15 | 54.33 ± 6.24 | 159.57 ± 6.37 | 75.79 ± 9.69 | 76.25 ± 10.03 | 29.85 ± 4.16 | 30.02 ± 4.19 |
| 2 | CR | 13 | 53.00 ± 9.00 | 162.50 ± 8.74 | 78.76 ± 11.01 | 78.52 ± 11.37 | 29.71 ± 2.06 | 29.61 ± 2.27 |
| 3 | CR & PE | 15 | 54.27 ± 7.12 | 158.67 ± 6.90 | 77.12 ± 11.22 | 77.69 ± 12.90 | 30.64 ± 4.11 | 30.89 ± 4.94 |
Abbreviations: C, control group; CR, cardiac rehabilitation group; PE, pulmonary exercise group; BMI, body mass index.
a Values are expressed as mean ± SD unless otherwise indicated.
According to Table 2, the lowest mean weight (75.79 kg) belonged to the control group, followed by the rehabilitation-respiratory exercise (77.12 kg) and rehabilitation (78.79 kg) groups. After the administration of the protocol, the groups had slight changes, but the weight trend followed the same order.
The highest body mass index (BMI) belonged to the rehabilitation-respiratory exercise group (30.64 kg/m2), followed respectively by the control (29.85 kg/m2) and rehabilitation groups (29.71 kg/m2). At the end of the protocol, the rehabilitation-respiratory exercise group had the highest value (30.89 kg/m2), followed by the control (30.02 kg/m2) and rehabilitation (29.61 kg/m2) groups, respectively.
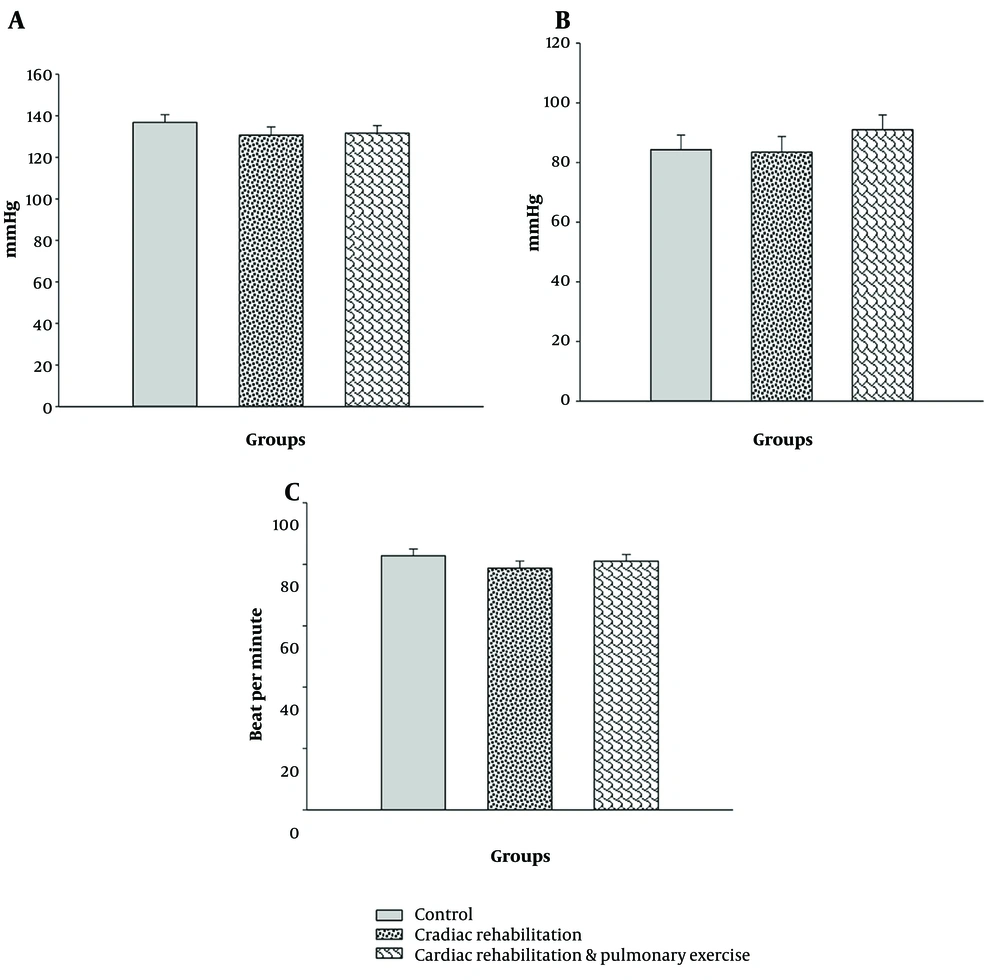
The results of the analysis of covariance showed no significant difference in the resting heart rate at the end of the research period despite the lower heart rate due to exercises (P > 0.05; Figure 1A). Moreover, the resting systolic and diastolic BPs were not influenced by the rehabilitation or rehabilitation-respiratory exercises (P > 0.05; Figure 1B and C).
Mean values of the groups after eliminating the effect of the covariate. A, resting systolic blood pressure (mmHg) (covariates appearing in the model were evaluated at the following value: Presystolic pressure = 137.37); B, resting diastolic blood pressure (mmHg) (covariates appearing in the model were evaluated at the following value: Prediastolic pressure = 83.65); C, resting heart rate (count/s) (covariates appearing in the model were evaluated at the following values: Pre-heart rate = 87.70). No significant difference was observed among the groups (P > 0.05).
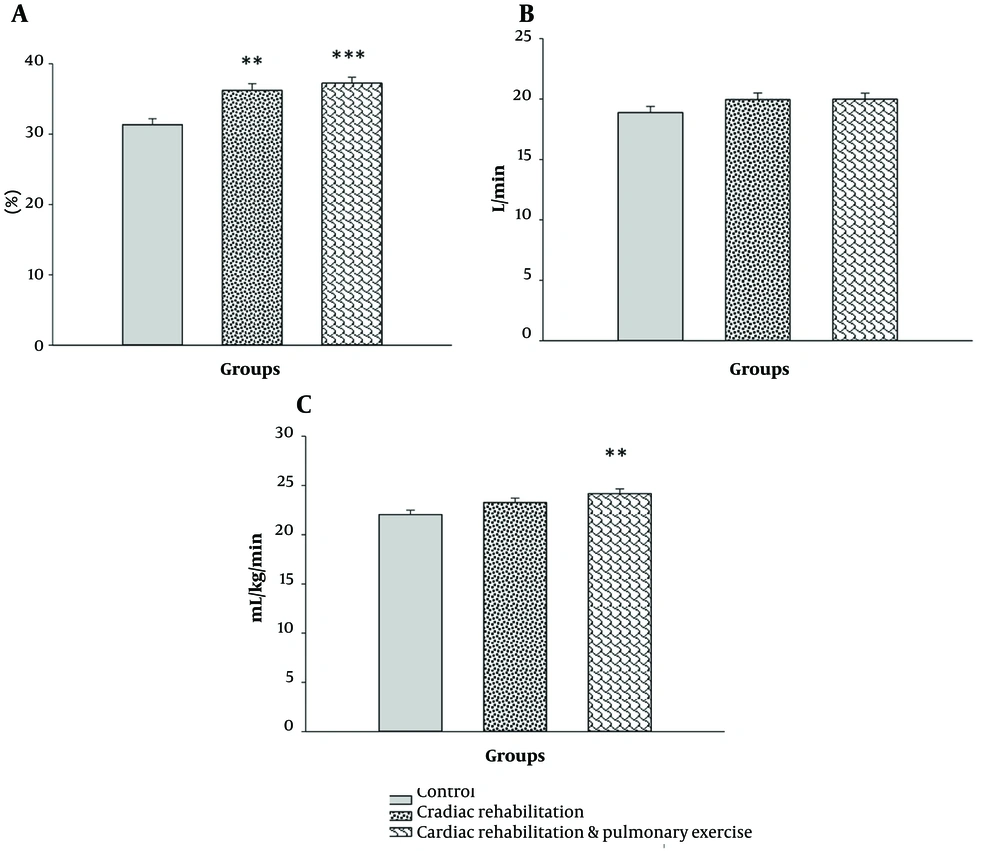
The injection fraction showed a significant difference among the groups (F2,39 = 13.824, P = 0.000, η2 = 0.415). After the elimination of the covariance effect, the mean values of the rehabilitation (P = 0.002) and rehabilitation-respiratory (P = 0.000) groups were significantly higher than those of the controls. However, the rehabilitation and rehabilitation-respiratory groups showed no significant difference (P > 0.05; Figure 2A). The ventilatory threshold was not affected by the exercises (P > 0.05; Figure 2B).
The mean values of the groups after removing the effect of the covariate (pretest). A, ejection fraction (%) (covariates appearing in the model were evaluated at the following value: Pre-ejection fraction = 31.67); B, ventilatory threshold (L/min) (covariates appearing in the model were evaluated at the following value: Pre-ventilatory threshold = 19.02); C, maximal oxygen uptake (mL/kg/min) (covariates appearing in the model were evaluated at the following value: Pre-VO2 max = 21.70). **: A significant difference with the control group at P > 0.01. ***: A significant difference with the control group at P > 0.001.
Concerning VO2 max, the analysis of covariance exhibited significant differences between the groups (F2,39 = 5.224, P = 0.010, η2 = 0.211). A comparison of the mean values with Bonferroni's correction showed that only the mean of the rehabilitation-respiratory group was significantly higher than that of the controls (P = 0.008; Figure 2C).
5. Discussion
The results indicated that rehabilitation exercises did not affect resting heart rate. Tulppo et al. and Billman et al. expressed that aerobic exercise can reduce the heart rate in inactive people due to the increase in stroke volume and the enhancement in vagal tone on the sinoatrial atrium node (18, 19). Although the training duration in their studies was similar to the current research, the number of training sessions was different, i.e., 6 training sessions per week in their study vs. 3 sessions in the present research. Previous studies have shown the significance of rehabilitation programs for HF patients as they increase their exercise capacity, hemodynamics, vascular and autonomic system, and quality of life while reducing depression, risk factors of cardiovascular diseases, mortality, hospitalization, and heart attack (20). The intensity and type of exercises, as well as the subjects, were also different. Cornelissen et al. and Grassler et al. mentioned the intensity and type of exercise among the effective factors of exercise-induced adaptations (21, 22). Perhaps home exercises were not intense enough. Furthermore, the resting heart rate of the participants in this research was around 80 beats per minute, which is almost within the normal range (23-25). Exercise usually approximates disorders to the normal range while having no specific effect on people who are already within the normal range.
Blood pressure is a key factor in blood circulation. Blood pressure beyond the normal limit indicates vascular resistance to blood circulation. Aerobic exercise improves the endothelial function, neurohormonal structure, BP, exercise capacity, and quality of life of patients due to its anti-remodeling and anti-inflammatory effects, as well as reducing mortality and complications in cardiovascular patients (6). Specifically, aerobic exercise decreases systolic and diastolic BP in hypertensive adults, regardless of whether exercise variables improve (26). In this study, similar to rehabilitation exercises, respiratory exercises caused a slight nonsignificant reduction in BP. The results of this research revealed that rehabilitation exercises at home or rehabilitation exercises combined with respiratory exercises for 8 weeks (3 times a week) could not reduce systolic or diastolic BP. The average systolic and diastolic BP of the subjects was 133.16 and 86.42 mmHg, respectively. Although these values are higher than the normal range, they are in the primary stage of BP (level 1) (27). Lack of hypertension seems to be a reason for the lack of significant variation in the patients' BP. Most studies have addressed the effect of endurance exercises on people with hypertension. Based on Cornelissen et al., exercise can reduce high systolic BP in older subjects (21). In this regard, both high-intensity interval training (HIIT) and moderate-intensity long-term continuous training lower systolic BP in hypertensive adults, with HIIT training having a greater impact on diastolic BP (28).
The ventilatory threshold increased as a result of rehabilitation exercises in HF subjects. The addition of respiratory exercises further changed the HF value, although these changes did not cause significant differences. The ventilatory threshold is associated with the ability to perform sports activities. Although it was previously shown to be related to VO2 max (29), it is related to blood lactate concentration more (30, 31). Our results demonstrated that VO2 max increased as a result of rehabilitation and respiratory exercises in people with HF, while rehabilitation alone could not generate these changes, probably due to the strengthening of the respiratory muscles. This strengthening, especially the inspiratory muscles, has been shown to increase the ability to perform sports activities, supply more oxygen to active skeletal muscles, and increase VO2 max to some extent. According to Sadek et al., performing high-intensity interval aerobic exercises along with inspiratory exercises can improve the function of respiratory muscles, exercise performance, and quality of life of patients with HF and weak inspiratory muscles (32). Maximal oxygen consumption is a function of cardiac output and arteriovenous oxygen difference. Therefore, an increase in any of the mentioned factors can affect maximal oxygen consumption. Cardiac output is the product of heart rate and stroke volume. A rise in the injection fraction can affect the stroke volume (33, 34). The injection fraction is reduced in some patients with HF (35, 36), but it has been reported to be preserved in about half of those with HF (37). In this research, the rehabilitation exercise increased the ejection fraction, whose effect can be enhanced when combined with respiratory exercises. A factor of increase in VO2 max in the rehabilitation and respiratory group might be the rise in the ejection fraction, which may not be sufficiently increased in the rehabilitation group.
Overall, the current research results revealed that 8 weeks of home rehabilitation exercise 3 times a week did not significantly alter the cardiac and respiratory function factors. It only significantly increased the injection fraction, which is an important factor. Although the stroke volume was not measured, it will also increase this factor. When combined with the respiratory exercise, home rehabilitation exercise enhanced the VO2 max of HF patients, in addition to their injection fraction. The combination of respiratory and rehabilitation exercises may exert more positive impacts. Finding better outcomes and an exercise with more beneficial effects on these patients requires further investigation and administration of exercises differing in terms of exercise intensity, duration, and type. Moreover, note that the subjects had relatively normal resting heart rates and systolic and diastolic BP. Thus, subjects with different conditions may show different results. This research was conducted on HF patients aged 40 - 60 years; although recommendations were given to the patients, their diet was not controlled. Dietary control may help improve the outcomes. Furthermore, the approach of this research was to provide rehabilitation and respiratory exercises at home for patients who did not have the opportunity to go to clinics. As a result, some aspects may have needed to be controlled properly, although the effect of these exercises was observed on some cardiovascular functions.