1. Background
Multiple sclerosis (MS) is the most common chronic autoimmune disease affecting the central nervous system (CNS), leading to varying degrees of neurodegeneration and neurological dysfunction, including cognitive impairment (1, 2). Cognitive dysfunction in MS affects information processing, remote memory, visual learning, and executive function, all of which are crucial for patients’ quality of life and social participation (3, 4). Magnetic resonance imaging (MRI) is the preferred modality for evaluating MS progression, although discrepancies between MRI findings and clinical symptoms are common (5). Typical MRI lesions in MS occur in periventricular, juxtacortical, infratentorial regions, and the corpus callosum (6). While the relationship between brain region involvement and cognitive changes has been explored, the precise mechanisms underlying cognitive impairment in MS remain unclear (7).
2. Objectives
This study aims to investigate the association between the distribution of cerebral lesions and cognitive dysfunction in patients with relapsing-remitting MS (RRMS).
3. Methods
This cross-sectional study enrolled 100 participants (73 females) with RRMS, aged 18 - 55 years, who underwent brain MRI at Besat Clinic Imaging Center between March 2020 and March 2024. Exclusion criteria included patients older than 55 years, Body Mass Index (BMI) over 35 kg/m², history of major depressive disorder, recent head trauma, seizures, substance or alcohol abuse, or shock within the past six months. After explaining the study objectives and obtaining written informed consent, participants underwent cognitive assessment using the montreal cognitive assessment (MoCA) within one week of MRI. The MoCA is a 30-point test that evaluates several cognitive domains, including memory, executive function, attention, language, abstraction, and orientation (8). A score of 26 or above is considered normal, and patients were classified into two groups: (1) Cognition impaired (CI) with a MoCA score of 25 or less; and (2) cognition preserved (CP) with scores of 26 or higher. Participants were further grouped by disease duration: Less than five years, 5 - 10 years, and more than 10 years. The history of comorbidities, such as diabetes mellitus and hypertension, was also recorded. Brain MRIs were performed using a 1.5 Tesla Siemens MRI system, with sequences including T1-weighted, T2-weighted, and FLAIR, along with T1 post-contrast sequences for clinically indicated cases. Two expert radiologists evaluated and recorded the location and number of demyelinating plaques, categorized into frontal, parietal, temporal, occipital lobes, and corpus callosum. Descriptive statistics were shown, including means, standard deviations, and tables. Quantitative data were analyzed employing parametric or non-parametric tests, depending on the normality of the data distribution. A correlation test was applied to evaluate the relationship between the variables. Data were analyzed using SPSS version 26, with a significance level set at P < 0.05.
4. Results
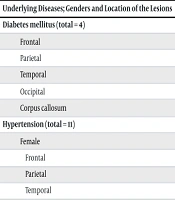
The demographic characteristics of the study population are shown in Table 1 and Table 2. Analysis revealed a significant correlation between plaque location and cognitive impairment. Specifically, plaques in the frontal lobe and corpus callosum were significantly associated with cognitive dysfunction (P = 0.006 for the frontal lobe, P = 0.004 for the corpus callosum) (Table 3). No significant association was found between disease duration and cognitive impairment (Table 4). Further analysis of patients with diabetes (n = 4) and hypertension (n = 11) revealed no significant differences in plaque distribution between these patients and those without these comorbidities (Table 5).
| Parameters | Total | CP | CI |
|---|---|---|---|
| Age | 38.3 ± 8.2 | 36.2 ± 7.3 | 42.1 ± 6.8 |
| Male/female | 27/73 | 21/55 | 6/18 |
| Disease duration (y) | |||
| Male | 11.3 ± 8.6 | 11.2 ± 8.6 | 10.3 ± 9.2 |
| Female | 11.8 ± 7 | 11.9 ± 7.1 | 10.6 ± 6.9 |
Abbreviations: CP, cognition preserved; CI, cognition impaired.
| Variables | No. (%) |
|---|---|
| Gender | |
| Male | 27 (27) |
| Female | 73 (73) |
| Education | |
| Below diploma | 33 (43) |
| Diploma | 40 (40) |
| University degree | 27 (27) |
| Diabetes mellitus (4 females, 0 male) | 4 (4) |
| Hypertension (12 females, 3 males) | 15 (15) |
| Location of lesions | CP | CI | P-Value |
|---|---|---|---|
| Frontal | 26 (16) | 20 (13) | 0.006 |
| Parietal | 33 (21) | 17 (11) | 0.2 |
| Temporal | 10 (6) | 4 (2) | 0.09 |
| Occipital | 7 (4) | 3 (2) | 0.1 |
| Corpus callosum | 20 (13) | 17 (11) | 0.004 |
| Total | 96 (61) | 61 (39) | - |
Abbreviations: CP, cognition preserved; CI, cognition impaired.
a Values are expressed as No. (%).
| Durations and Location of Lesions | CP | CI | P-Value |
|---|---|---|---|
| Less than 5 years (total = 55) | |||
| Frontal | 13 (8) | 11 (7) | 0.2 |
| Parietal | 19 (12) | 10 (6) | 0.08 |
| Temporal | 6 (4) | 3 (2) | 0.07 |
| Occipital | 5 (3) | 2 (1) | 0.08 |
| Corpus callosum | 12 (8) | 9 (6) | 0.09 |
| 5 - 10 years (total = 31) | |||
| Frontal | 8 (5) | 5 (3) | 0.3 |
| Parietal | 9 (6) | 4 (2) | 0.07 |
| Temporal | 3 (2) | 1 (1) | 0.2 |
| Occipital | 1 (1) | - | - |
| Corpus callosum | 5 (3) | 5 (3) | 0.9 |
| More than 10 years (total = 14) | |||
| Frontal | 5 (3) | 4 (2) | 0.5 |
| Parietal | 5 (3) | 3 (2) | 0.4 |
| Temporal | 1 (1) | - | - |
| Occipital | 1 (1) | 1 (2) | 0.9 |
| Corpus callosum | 3 (2) | 3 ( 2) | 0.9 |
Abbreviations: CP, cognition preserved; CI, cognition impaired.
a Values are expressed as No. (%).
| Underlying Diseases; Genders and Location of the Lesions | CP | CI | P-Value |
|---|---|---|---|
| Diabetes mellitus (total = 4) | 0.1 | ||
| Frontal | 3 | 2 | |
| Parietal | 2 | 1 | |
| Temporal | - | - | |
| Occipital | - | - | |
| Corpus callosum | 1 | - | |
| Hypertension (total = 11) | 0.2 | ||
| Female | |||
| Frontal | 5 | 3 | |
| Parietal | 2 | 2 | |
| Temporal | 1 | 1 | |
| Occipital | - | - | |
| Corpus callosum | 3 | 2 | |
| Male | |||
| Frontal | 2 | 1 | |
| Parietal | 1 | - | |
| Temporal | - | - | |
| Occipital | - | - | |
| Corpus callosum | 2 | 1 |
Abbreviations: CP, cognition preserved; CI, cognition impaired.
5. Discussion
In this study, we found a significant association between the accumulation of demyelinating plaques in the frontal lobe and corpus callosum and cognitive dysfunction in patients with MS. Specifically, patients classified as CI showed a higher number of plaques in these regions compared to those with CP. These findings support the hypothesis that the localization of demyelinating plaques, particularly in critical regions involved in cognitive processing, may contribute to the cognitive deficits observed in MS. Our results align with previous studies that have investigated the role of specific brain regions in cognitive decline among MS patients (9). The frontal lobe is essential for executive functions, which include planning, problem-solving, and cognitive flexibility, while the corpus callosum facilitates interhemispheric communication. Lesions in these areas can disrupt critical neural pathways, potentially leading to the cognitive impairment observed in our CI group (10, 11). These findings suggest that MRI markers in the frontal lobe and corpus callosum may serve as important indicators for identifying patients at higher risk of cognitive decline. Several studies have previously highlighted the role of frontal lobe pathology in MS-related cognitive dysfunction. For example, Morgen et al. (12) demonstrated that MS patients with frontal lobe lesions exhibited poorer performance on tasks requiring executive function. Our findings are consistent with this, underscoring the vulnerability of frontal brain regions in MS. Similarly, previous research has shown that damage to the corpus callosum is linked to deficits in processing speed and interhemispheric transfer, which are key components of cognitive function (13). The significant association between corpus callosum plaques and cognitive impairment in our study supports these observations. Interestingly, while we observed a significant correlation between plaque accumulation in the frontal lobe and corpus callosum with cognitive dysfunction, no such relationship was found for other brain regions, such as the parietal, temporal, or occipital lobes. This may indicate a particular vulnerability of frontal-subcortical pathways in MS-related cognitive impairment, a hypothesis that has been supported by several neuroimaging studies. Paul (14), for instance, reported a similar lack of association between parietal and occipital lobe plaques and cognitive dysfunction, suggesting that cognitive impairment in MS may not be solely related to the overall burden of demyelinating lesions but rather to their specific location and impact on key cognitive networks. Our study also explored the relationship between disease duration, comorbidities (diabetes mellitus and hypertension), and cognitive status, with no significant associations found. This finding is in line with earlier studies that have reported mixed results on the influence of comorbidities in MS.
Recently, studies have focused on different domains of cognitive impairment and subdivided subjects based on the affected domain (e.g., processing speed, memory, executive functioning/working memory, and attention) (15). Evaluation of imaging characteristics with regard to the affected cognitive domain can elucidate the potential involved brain region for each. While some evidence suggests that cardiovascular comorbidities, such as hypertension and diabetes, may exacerbate MS pathology due to their impact on cerebral vascular health (16, 17), our study did not find a significant effect of these factors on plaque distribution or cognitive outcomes. This could be due to the small sample size of patients with comorbidities in our cohort, particularly diabetes, which limits our ability to draw definitive conclusions. Future studies with larger, more diverse samples may be necessary to fully explore the impact of these comorbid conditions on MS progression and cognitive decline. Our findings also raise questions about the potential underlying mechanisms contributing to cognitive impairment in MS. Although plaque accumulation in the frontal lobe and corpus callosum was associated with cognitive dysfunction, it is unlikely that lesion location alone accounts for the full spectrum of cognitive changes seen in MS. Growing evidence suggests that additional factors, such as brain atrophy, microstructural damage to normal-appearing white matter, and gray matter involvement, may play crucial roles in cognitive and physical decline (18). For instance, diffuse axonal damage, cortical thinning, and hippocampal atrophy have all been implicated in the pathophysiology of cognitive impairment in MS. As highlighted by Granberg et al. (19), volumetric MRI assessments and advanced techniques such as diffusion tensor imaging (DTI) and magnetization transfer imaging (MTI) could provide more comprehensive insights into the structural changes associated with cognitive dysfunction. Moreover, recent studies have suggested that inflammatory processes, including oxidative stress and mitochondrial dysfunction, may contribute to both lesion formation and neurodegeneration in MS (20). These factors may influence not only plaque formation but also broader cortical and subcortical brain changes that affect cognitive function. Given the complex interplay of these mechanisms, it is likely that cognitive dysfunction in MS is a multifactorial process, with plaque location being just one of several contributing factors. Future research should aim to integrate both lesion-based and non-lesion-based MRI markers, along with biomarkers of inflammation and neurodegeneration, to better understand the full scope of cognitive impairment in MS. It is also important to acknowledge the limitations of this study. First, the cross-sectional design limits our ability to draw conclusions about the temporal relationship between plaque accumulation and cognitive decline. Longitudinal prospective studies with larger sample sizes are needed to determine whether the presence of plaques in the frontal lobe and corpus callosum can predict future cognitive deterioration. Additionally, while we focused on RRMS, the findings may not be generalizable to other forms of MS, such as primary progressive or secondary progressive MS, which are known to have distinct patterns of neurodegeneration and cognitive decline.
Another limitation of our study is the relatively small sample size, particularly regarding the number of patients with comorbid conditions such as diabetes and hypertension. The small number of comorbid cases limits the statistical power of our analysis and may have contributed to the lack of significant findings related to these variables. Furthermore, while we used the MoCA to evaluate cognitive function, this test, though widely used, may not capture the full complexity of cognitive impairment in MS. A more comprehensive neuropsychological battery could provide additional insights into the specific cognitive domains affected by MS and their relationship to brain pathology. Finally, one of our major limitations while conducting the current study was the treatment strategy during which we assessed cognition and imaging. Different medications might affect cognition, which prompts precise attention in future treatment-oriented studies. Despite these limitations, our study adds to the growing body of literature on the neural correlates of cognitive dysfunction in MS. The significant association between plaque location in the frontal lobe and corpus callosum and cognitive impairment highlights the importance of regional brain involvement in MS. These findings suggest that MRI could potentially be used not only for tracking disease progression but also for identifying patients at risk for cognitive decline. By combining lesion mapping with other advanced neuroimaging techniques and clinical assessments, future studies may help refine cognitive prognostication in MS and guide the development of targeted therapeutic interventions aimed at preserving cognitive function.
5.1. Conclusions
This study suggests that demyelinating plaques in the frontal lobe and corpus callosum are associated with cognitive impairment in MS. Prompt identification and individualized therapeutic strategies may facilitate the deferment of progressive cognitive deterioration. Additionally, future research with a prospective longitudinal approach and larger sample size focusing on different domains of cognition could provide a better understanding of the progression of cognitive dysfunction in MS patients.