1. Context
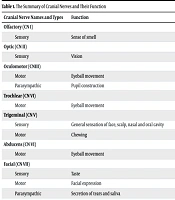
The peripheral nervous system (PNS) consists of the remaining nerve pathways outside the brain and spinal cord, which include 12 pairs of cranial nerves and 31 pairs of spinal nerves (1). Among these, the cranial nerves are numbered I to XII in Roman numerals, reflecting their sequential emergence from the rostral (front) to the caudal (back) regions of the brainstem (2). With the exception of cranial nerves I and II, which are considered extensions of brain tissue, the remaining ten cranial nerves (III-XII) originate from the brainstem and possess specific nuclei within it (Table 1). While this traditional classification provides a framework for understanding cranial nerves, the scientific reality is more intricate, leading to ongoing debates regarding the categorization and identification of the pathways of distinct cranial nerve fibers, as well as the existence of lesser-known structures like the terminal nerve, also referred to as cranial nerve zero (CN0) (3).
| Cranial Nerve Names and Types | Function | Explorer | Origin |
|---|---|---|---|
| Olfactory (CN I) | |||
| Sensory | Sense of smell | Caspar Bartholin-1611 | Olfactory epithelium |
| Optic (CN II) | |||
| Sensory | Vision | Constanzo Varolio- (1543 - 1575) | Retina |
| Oculomotor (CNIII) | Gustav Fritsch- 1878 | Brain stem | |
| Motor | Eyeball movement | ||
| Parasympathic | Pupil construction | ||
| Trochlear (CN VI) | |||
| Motor | Eyeball movement | Vesalius – (1514 -1564) | Brain stem |
| Trigeminal (CN V) | Winslow- 1732 | Brain stem | |
| Sensory | General sensation of face, scalp, nasal and oral cavity | ||
| Motor | Chewing | ||
| Abducens (CN VI) | |||
| Motor | Eyeball movement | Sommerring-1778 | Brain stem |
| Facial (CN VII) | Sommerring-1778 | Brain stem | |
| Sensory | Taste | ||
| Motor | Facial expression | ||
| Parasympathic | Secretion of tears and saliva | ||
| Vestibulocochlear (CN VIII) | |||
| Sensory | Hearing and balance | Galen- (129 - 216 AD) | Brain stem |
| Glossopharyngeal (CN IX) | Haller-1753 | Brain stem | |
| Sensory | Taste and sensation back of tongue | ||
| Motor | Swallowing and speech | ||
| Parasympathic | Secretion of saliva | ||
| Vagus (CN X) | Bartholin-1611 | Brain stem | |
| Sensory | Taste and sensation epiglottis | ||
| Motor | Swallowing and speech | ||
| Parasympathic | Muscle construction of abdominal organs and secrete digestive fluids | ||
| Accessory (CN XI) | |||
| Motor | Head and shoulder movement | Willis- (1621 - 1675) | Brain stem-Spinal cord |
| Hypoglossal (CN XII) | |||
| Motor | Head and shoulder movement | Winslow-1732 | Brain stem |
| CN XIII/ CN0 | |||
| Unknown | Unknown | Fritsch | Olfactory tract |
Abbreviation: CN, cranial nerve.
2. History of the Discovery of Cranial Nerves
The earliest references to cranial nerves can be traced back to medical writings by Alexandrian physicians, such as Herophilus (circa 290 BC) and Erasistratus (280 BC). Most terminologies emerged during the 17th and 18th centuries (4). The nomenclature for the twelve cranial nerves was established through various anatomical discoveries made by renowned anatomists, from Galen to Von Sommerring (5). A Roman and Greek physician, surgeon, and philosopher, Aelius Galenus or Claudius (129 - 200 AD), contributed significantly to the field of nerve anatomy, providing crucial insights that benefited physicians. Similarly, Abu Ali Sina (Ibn Sina) proposed that there are nine pairs of cranial nerves, correlating with the number of foramina at the base of the skull from which they emerge. In his "Book of Law", he detailed the vagus nerve and its extensive connections to the heart, lungs, respiratory tract, digestive system, and even the ears. Seyyed Isma'il Jurjani (1042 - 1137 AD), a prominent figure in Islamic and Iranian traditional medicine, wrote extensively about cranial nerves in his work "Zakhireh Khwarazm Shahi", which stands alongside Abu Ali Sina's "Canon of Medicine" (6).
In 1664, Sir Thomas Willis (1621 - 1675), known as the "founder of clinical neuroscience", listed and illustrated nine cranial nerve pairs, identifying the olfactory nerves as the first pair. He also counted the trochlear, trigeminal, and abducens nerves individually, numbering them IV to VI in their current order. Caspar Bartholin the Elder (1585 - 1629), a Danish physician and anatomist, was one of the first to accurately number the olfactory and trochlear nerves before Willis (7). In 1632, von Sömmerring (1755 - 1830), a German physician and anatomist, described the organization of cranial nerves in his doctoral dissertation, which remains relevant today. The discourse on naming and numbering cranial nerves continued, with all anatomists acknowledging the existence of twelve pairs, but debates arose about an additional nerve (8).
The concept of a "zero" or "thirteenth" nerve, known as the terminal nerve (NT), was first discussed by Fritsch. Gustav Theodor Fritsch (1838 - 1927) identified it in sharks. This discovery complicated the counting of cranial nerves. The CN0 was later described in detail by Pinkus in 1895, and subsequently by Locy, who depicted it in selachians and referred to it as NT (9, 10). In 1987, Demsky and Schwanzel-Fukuda designated this nerve as cranial nerve zero, noting its rostral connections to all other cranial nerves. Due to the absence of a zero in Greek numeral systems, it was also called "Nulla nerve" (11). In 1914, Brookover and Johnston formally labeled it as the "0" pair of cranial nerves, and in 2007, CN0 was identified as a common finding in adult humans (12, 13).
3. Gross Anatomy
The CN0 nerve is situated anteriorly to the other cranial nerves on both sides, appearing as a microscopic network of unmyelinated peripheral nerves within the subarachnoid space. It covers the rectus gyrus and is located near the cribriform plate, extending posteriorly towards the olfactory trigone and the internal olfactory gyrus, linking the most anterior forebrain derivatives to nasal and olfactory structures (14).
4. Development
The origins of CN0 remain largely enigmatic during embryonic development. Establishing the terminal nerve and its fibers is challenging due to their migratory growth patterns and the differentiation of tissues forming the embryonic olfactory placode (15). Some researchers propose that CN0 develops from the olfactory placode, while others suggest it arises from the neural crest, given its location at the interface of migrating cranial neural crest cells. This nerve may result from the fusion of various migrating cells, with the neural crest potentially contributing to a subset of gonadotropin-releasing hormone (GnRH)-secreting neurons (16).
5. Physiological Function
Research indicates that CN0 fibers differ from those of the olfactory and vomeronasal nerves, suggesting a possible role in pheromone perception or other olfactory functions. Its fibers travel near the cribriform plate to regions like the olfactory trigone and medial olfactory gyrus (17). Although its exact function remains debated, it is believed to influence autonomic functions and may play a role in mate selection and reproductive behavior (18). The CN0, also known as the terminal nerve, is significant in regulating reproductive hormones, particularly GnRH, which stimulates the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the adenohypophysis, regulating sex steroid production and secretion. This nerve is located on the ventral surface of the human brain and is closely associated with the olfactory nerve (CNI). It is hypothesized to have neuromodulatory effects on GnRH and the blood vessels and glands of the nasal mucosa, indicating a role in developing the hypothalamic-pituitary-gonadal (HPG) axis and influencing human sexual behavior (15, 19-22).
The GnRH component of CN0 may enhance the detectability of pheromones through its neuromodulatory effects on the olfactory epithelium. Its anatomical connections to various neuroanatomical structures, including the hypothalamus and nasal mucosa, provide pathways to the limbic system. Within the hypothalamus, particularly in the preoptic and infundibular nuclei, the "kisspeptin neuronal network" (KP) regulates puberty and reproductive functions. Kisspeptin neuronal network neurons primarily induce GnRH secretion from the hypothalamus, which in turn influences LH and FSH secretion and the synthesis and release of sex steroids from the gonads. The potential neuromodulatory function of CN0 in sexual behavior through GnRH is intriguing, as it projects to the nasal mucosa, amygdala, and hypothalamus (21). If these projections reach the preoptic and infundibular nuclei, they could represent an afferent component to the KP neurons regulating GnRH secretion and, consequently, human sexual behavior and function. This hypothesis warrants further scientific investigation (23-26).
6. Clinical Implications
The clinical significance of CN0 lies in its potential association with Kallmann syndrome (KS), an inherited disorder characterized by hypogonadotropic hypogonadism (HH) and hyposmia or anosmia in both sexes. Research suggests that disruptions in the normal migration of basal forebrain GnRH cells during embryonic development may lead to the hypogonadism observed in KS (27). Additionally, mutations in the kisspeptin 1 gene (KISS1), which can result in hypogonadotropic hypogonadism or precocious puberty, are noteworthy (28). This gene is expressed in the central nervous system (CNS), particularly in the hypothalamus, but also in regions such as the amygdala, caudate, cingulate, globus pallidus, hippocampus, medial and superior frontal gyri, nucleus accumbens, parahippocampal gyrus, substantia nigra, putamen, and thalamus (29). Furthermore, kisspeptin plays a crucial role in fear, anxiety, reward pathways, negative emotions, and olfaction, potentially influencing syndromes related to CNS structures and mood (30).
7. Conclusions
The CN0 is a recognized anatomical and functional nerve structure, yet it is often overlooked in medical literature and educational resources, which typically reference only twelve cranial nerves, as seen in texts like Gray's Anatomy. Recent studies affirm that CN0 is a well-established neural structure with significant implications for the development of the GnRH system and human reproductive neurophysiology. Additionally, macroscopic dissections of human cadavers (including adult and fetal specimens) have confirmed the consistent presence of the terminal nerve. The nerve is located as a small, delicate bundle of unmyelinated fibers just anterior to the olfactory nerve (CN I) and is often not recognized in routine dissection due to its small size and delicate texture. Therefore, it may be necessary to reconsider the numbering of cranial nerves to include this nerve. Cadaver examinations should be conducted to validate the presence of CN0.