1. Background
The Acacia genus, which includes numerous species of trees and shrubs, is well-known for its rich phytochemical profile and broad geographic distribution across regions such as Senegal, the Western Sahara, tropical Africa, and southern Iran (1, 2). These species have been widely used in traditional medicine due to their diverse therapeutic properties. Among the notable bioactive compounds found in many Acacia species is N,N-dimethyltryptamine (DMT), a naturally occurring psychoactive substance (3). While DMT is widely recognized for its hallucinogenic properties, recent research has increasingly focused on its potential therapeutic roles, particularly in addressing neurological disorders (4).
Neurodegenerative diseases, such as Alzheimer’s disease and depression, are becoming more prevalent and represent major global health challenges (5, 6). Contemporary therapeutic approaches emphasize not only symptom management but also the enhancement of the brain’s regenerative capabilities (7). Evidence suggests that small, controlled doses of DMT can stimulate the production of brain-derived neurotrophic factor (BDNF), a protein that plays a critical role in neuronal survival, differentiation, and synaptic plasticity (8, 9). Elevated BDNF levels have been linked to improvements in both cognitive performance and mood regulation. Furthermore, recent findings indicate that BDNF-supported neurogenesis may help slow the progression of neurodegenerative diseases such as Alzheimer’s and could provide novel strategies for treating conditions like treatment-resistant depression (10).
In parallel with these findings, an increasing body of neuropharmacological research has examined the neuroprotective effects of plant-derived compounds, including polyphenols and flavonoids, due to their antioxidative properties and ability to stimulate neurogenesis. For instance, studies on Ginkgo biloba and Curcuma longa (turmeric) have demonstrated their potential to enhance BDNF expression and promote neuronal survival (11-15). These studies offer a comparative framework for exploring similar neurogenic effects in lesser-studied medicinal plants such as Acacia oerfota (Vachellia oerfota).
2. Objectives
Despite its recognized traditional use for antioxidant and anti-inflammatory purposes, the neurological impacts of A. oerfota — specifically its influence on neurogenesis and BDNF expression — have not been thoroughly investigated. To address this knowledge gap, the present study examines the neurobiological properties of A. oerfota, with a particular focus on its potential to modulate cell cycle phases and BDNF gene expression in PC12 cells, a widely accepted in vitro model for neuronal differentiation. This study evaluates the effects of Acacia extract on PC12 cells by assessing both BDNF gene expression and cell cycle distribution across distinct phases. The results aim to offer foundational insights into the therapeutic potential of A. oerfota and its bioactive constituents, particularly DMT, in promoting brain plasticity and contributing to the development of novel interventions for neurodegenerative diseases.
3. Methods
3.1. Preparation of Acacia Extract
A hydroalcoholic extract from A. oerfota leaves was prepared at the School of Pharmacy, Iran University of Medical Sciences. Briefly, 20 grams of dried, powdered leaves were soaked in 20 mL of hydroalcohol solution (70% ethanol and 30% distilled water) and shaken at room temperature for 48 hours. The resulting mixture was filtered, concentrated using a rotary evaporator, and subsequently dried in an incubator to obtain a purified extract.
3.2. Cell Culture
The PC12 cell line, sourced from the Pasteur Institute Cell Bank (Tehran, Iran), was employed in this study. Cells were cultured in RPMI-1640 medium supplemented with L-glutamine, penicillin-streptomycin (1:100), and 10% fetal bovine serum (FBS) to ensure sterility and support cell proliferation. Cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2. Cryopreserved PC12 cells were thawed by rapid transfer from liquid nitrogen to a 37°C water bath, followed by gentle resuspension in warm culture medium to eliminate dimethyl sulfoxide (DMSO). Cells were centrifuged at 1000 rpm for 5 minutes, resuspended in fresh medium, and seeded into T25 flasks. Cell morphology, adhesion, and contamination were monitored under a light microscope. Media were replaced every 2 - 3 days to maintain an optimal environment, and cells were passaged upon reaching 80% confluence. Due to the sensitivity of PC12 cells to enzymatic treatments such as trypsin, mechanical detachment using a scraper was employed.
3.3. MTT Assay
The MTT assay was utilized to assess the cytotoxic effects of the hydroalcoholic extract of A. oerfota. PC12 cells were seeded at a density of 104 cells per well in a 96-well plate and incubated for 18 hours to allow cell adherence and recovery of normal morphology. Cells were serum-starved using medium containing 4% FBS for 6 hours before treatment. Subsequently, cells were exposed to various concentrations of the extract (0.01, 0.05, 0.1, 0.2, 0.4, 0.8, and 1.6 µg/mL) in medium containing 4% FBS for 24 and 48 hours. Control wells contained medium without the extract. After treatment, 20 µL of MTT solution (5 mg/mL) was added to each well and incubated for 3.5 hours. The supernatant was carefully removed, and 150 µL of DMSO was added to dissolve the formazan crystals. Optical density (OD) was measured at 570 nm using an ELISA reader, and the percentage of cell viability was calculated relative to the control group.
3.4. RNA Extraction, Quality Assessment, and cDNA Synthesis
3.4.1. RNA Extraction
Total RNA was extracted from treated cells using RNX-Plus reagent (Cinna Gen, Iran). After removing the culture medium, 1 mL of RNX-Plus was added to each well, and cells were detached by gentle pipetting. The suspension was transferred to microtubes, incubated at room temperature for 5 minutes, and mixed with 200 µL of chloroform. Following vigorous shaking, the mixture was centrifuged at 12,000 rpm for 15 minutes at 4°C. The aqueous phase was transferred to a new tube, mixed with isopropanol, and incubated on ice for 15 minutes. RNA was pelleted by centrifugation, washed with 75% ethanol, air-dried, dissolved in RNase-free water, and stored at -20°C.
3.4.2. RNA Quality and Quantification
RNA quality was assessed using 1.5% agarose gel electrophoresis, where high-quality RNA displayed distinct 28S and 18S rRNA bands. Concentration and purity were determined via NanoDrop spectrophotometry (Thermo Scientific, USA) using 260/280 nm and 260/230 nm absorbance ratios.
3.4.3. cDNA Synthesis
cDNA was synthesized from 1 µg of RNA using the RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, USA) with random hexamer primers, as per the manufacturer’s instructions. The synthesized cDNA was stored at -20°C for further analysis.
3.5. Quantitative PCR
The qPCR was conducted using SYBR Green Master Mix (Applied Biosystems, USA) on an ABI 7500 Real-Time PCR System. GAPDH served as the housekeeping gene for normalization. Reactions (20 µL final volume) included 7.5 µL master mix, 1 µL cDNA, and 0.5 µM primers, using the following conditions: Initial denaturation at 95°C for 5 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute.
3.6. Primer Efficiency and Standard Curve
Primer efficiency was evaluated using standard curves generated from five serial cDNA dilutions (1:10). Efficiency (E) was calculated as E = 10(-1/slope), with a slope of -3.3 indicating 100% efficiency. Primer sequences and specifications are detailed in Table 1.
| Genes and Sequence (5'→ 3') | Length | Tm | GC% | Self-complementarity | Self 3' Complementarity | Product Length |
|---|---|---|---|---|---|---|
| GAPDH | 79 | |||||
| F: CCGCATCTTCTTGTGCAGTG | 20 | 59.83 | 55 | 4 | 3 | |
| R: CGATACGGCCAAATCCGTTC | 20 | 59.42 | 55 | 6 | 2 | |
| BDNF | 107 | |||||
| F: GGCTCTCATACCCACTAAGATACATC | 26 | 60.35 | 46.15 | 4 | 4 | |
| R: CGGAAACAGAACGAACAGAAACAG | 24 | 60.78 | 45.83 | 2 | 1 |
Features of Primers Used in Standard PCR and Real-time PCR
3.7. Antioxidant Activity Measurement
Reactive oxygen species (ROS), naturally or externally generated, can damage cellular components. The DCFDA assay was used to detect ROS, where the compound fluoresces upon reaction with ROS. Fluorescence was measured at Ex/Em = 485/525 nm.
3.8. Statistical Analysis
To analyze data related to the MTT assay and determine IC50 concentration, GraphPad Prism version 6 was used. Statistical data from qPCR were evaluated using SPSS PASW Statistics version 22, applying linear regression, one-way ANOVA, independent t-tests, and bivariate correlation tests. All qPCR results were analyzed using the relative expression method 2– ∆∆Ct. All graphs were plotted using Excel 2013.
4. Results
4.1. MTT Assay Results
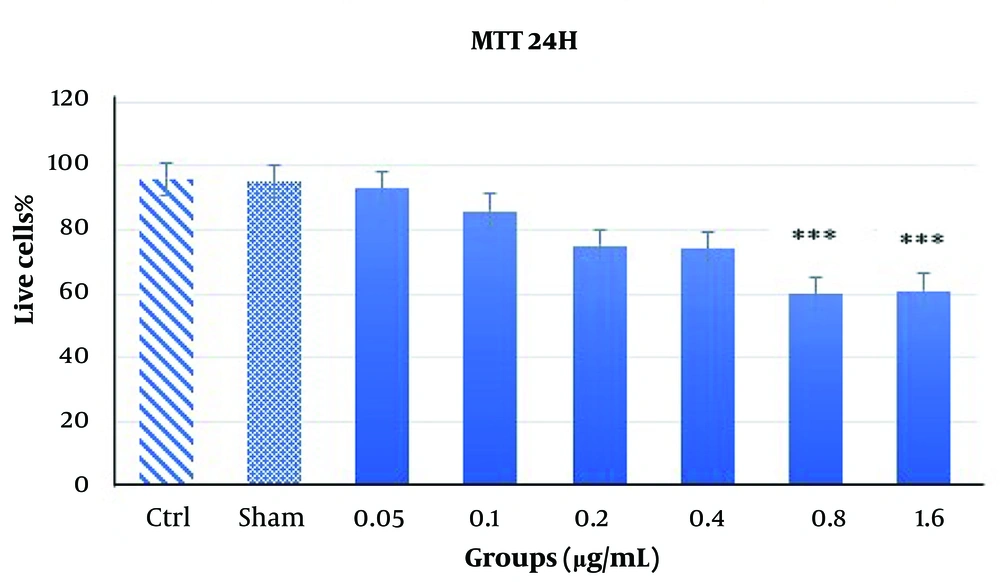
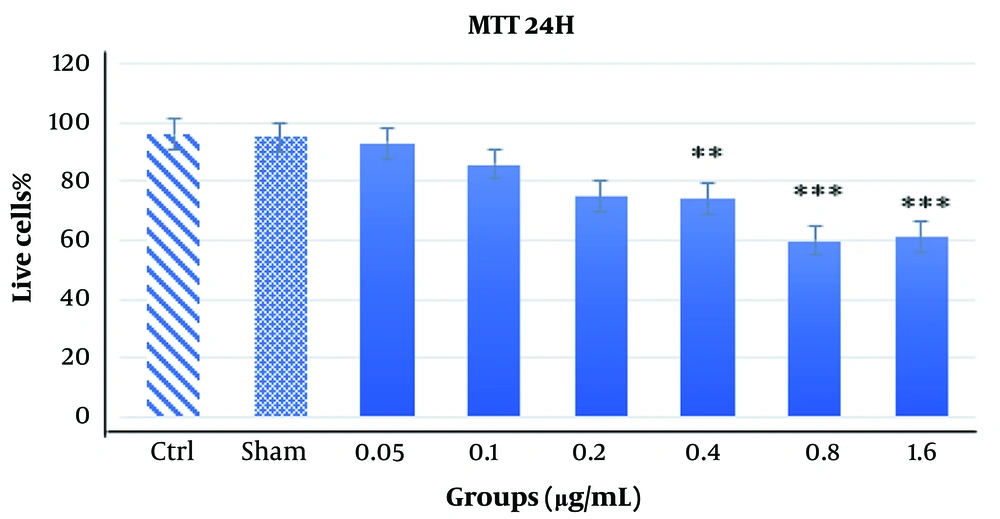
The results of the MTT assay at 24 and 48 hours with varying concentrations of Acacia extract are shown in Figures 1 and 2. At the 24-hour time point (Figure 1), most concentrations did not significantly reduce cell viability or exhibit cytotoxic effects. However, higher doses, particularly 1.6 µg/mL and 0.8 µg/mL, caused marked cytotoxicity and cell death, indicating a dose-dependent effect on cell viability. At 48 hours, the assay demonstrated increased toxicity levels (P < 0.001). While previously toxic doses (1.6 µg/mL and 0.8 µg/mL) remained cytotoxic, a concentration of 0.4 µg/mL, which showed no significant toxicity at 24 hours, exhibited significant cytotoxicity at 48 hours (P < 0.05), albeit to a lesser extent. This suggests that the cytotoxic effects of the Acacia extract may be both dose- and time-dependent, as demonstrated by the delayed toxicity observed at the 0.4 µg/mL concentration (Figure 2).
The MTT assay results over 24 hours with various doses of Acacia extract. The results indicate no significant reduction in cell viability at lower concentrations; however, a significant toxic effect ***P < 0.001 was observed at higher concentrations (0.8 μg/mL and 1.6 μg/mL), demonstrating a dose-dependent cytotoxic effect.
The results obtained from the MTT assay after 48 hours with different doses of Acacia extract. The MTT assay results at 48 hours reveal a time- and dose-dependent cytotoxic effect of Acacia extract on PC12 cells. A significant reduction in cell viability was observed at 0.4 μg/mL **P < 0.01, and a more pronounced toxic effect was detected at 0.8 μg/mL and 1.6 μg/mL ***P < 0.001.
4.2. Reactive Oxygen Species Production Results
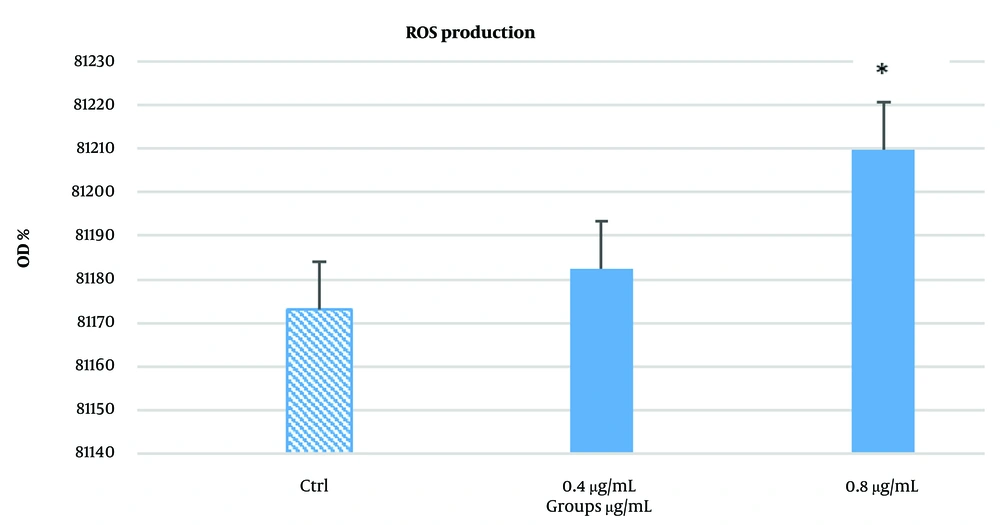
Based on the MTT assay results, two concentrations, 1.6 µg/mL and 0.8 µg/mL, were selected for analysis at the 48-hour time point, as they showed initial signs of efficacy. As shown in Figure 3, the 0.4 µg/mL dose did not significantly elevate ROS levels or induce apoptosis. However, the 0.8 µg/mL concentration caused a modest but significant increase in ROS production (P < 0.05).
The results obtained from the reactive oxygen species (ROS) assay. As shown in the chart, the 0.4 μg/mL dose did not significantly increase ROS production or induce apoptosis. In contrast, the 0.8 μg/mL dose caused a noticeable increase in ROS levels. *P < 0.05, though the difference was not highly significant.
4.3. Brain-Derived Neurotrophic Factor Gene Expression Analysis
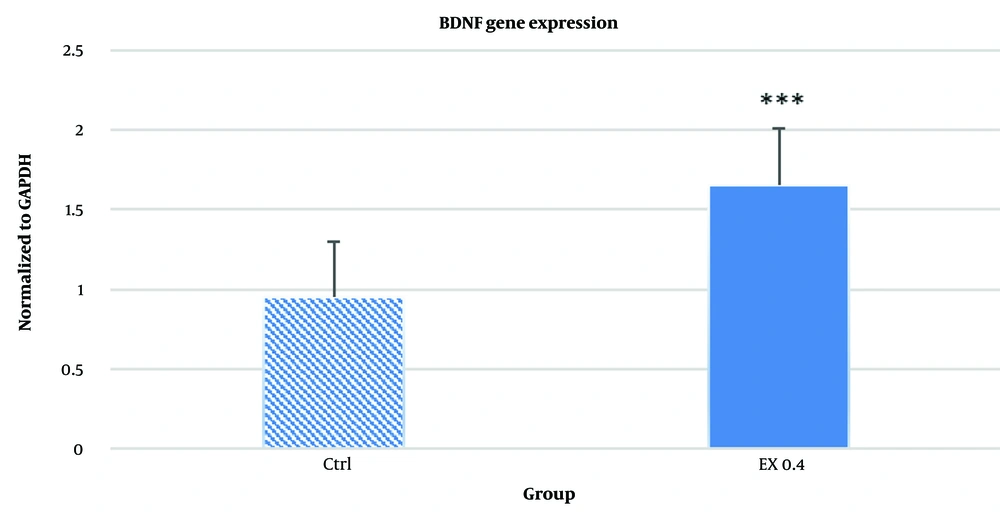
The 0.4 µg/mL dose was selected for comparison with the control group, as it exhibited the lowest toxicity in the MTT assay. As shown in Figure 4, the 0.4 µg/mL concentration significantly increased BDNF gene expression compared to the control group (P < 0.001). These findings highlight the potential of Acacia extract to enhance neurogenesis.
The results of the analysis of brain-derived neurotrophic factor (BDNF) gene expression. As shown in the chart, a significant increase in BDNF expression at the dose of 0.4 μg/mL of Acacia extract was observed compared to the control group ***P < 0.001, highlighting the neurogenic potential of the extract at this concentration.
5. Discussion
The findings of this study underscore the potential therapeutic properties of Acacia extract, particularly at low concentrations (0.4 µg/mL) and after short-term exposure (24 hours). These results suggest that bioactive compounds within the extract, including but not limited to N,N- DMT, may contribute to enhanced neurogenesis and thus offer promise in the treatment of neurodegenerative and psychological disorders. Previous research by Sadeghi et al. confirmed the presence of alkaloids and flavonoids in the hydroalcoholic extract of Boswellia, supporting its nootropic activities and memory-enhancing effects (2). One of the active constituents of Boswellia, α-pinene, has been shown to inhibit acetylcholinesterase activity, thereby enhancing cholinergic neurotransmission, which may contribute to cognitive improvement.
The therapeutic properties of Boswellia are primarily attributed to boswellic acids and their derivatives. Laboratory studies have demonstrated that these acids can inhibit the synthesis of pro-inflammatory mediators through selective inhibition of the enzyme 5-lipoxygenase. Immunohistochemistry studies have revealed inflammation in specific brain regions of patients with Alzheimer’s disease, and the administration of non-steroidal anti-inflammatory drugs (NSAIDs) has been shown to reduce memory decline in such cases (16-18). Therefore, the memory-enhancing effects of Boswellia may be partially explained by its anti-inflammatory action. Furthermore, Hayashi et al. reported that boswellic acids and related extracts activate protein kinases, which are likely critical for maintaining long-term synaptic changes (19). For instance, protein kinase A (PKA) influences synaptic vesicles and nerve terminals and activates the CREB signaling pathway, which regulates the expression of genes involved in long-term memory formation. This cascade enhances the sustained release of neurotransmitters at synapses and strengthens synaptic connectivity (20).
In the present study, treatment of PC12 cells with Acacia extract at a concentration of 0.4 µg/mL led to a 6.1% increase in BDNF gene expression, suggesting a potential role in promoting neurogenesis. Additionally, the SubG1 assay indicated a significant rise in DNA synthesis during the S phase, pointing to enhanced neurogenic rather than merely proliferative activity. It is important to note that the extract used in this study was derived from Acacia leaves and contained a range of bioactive compounds. Although DMT, known for its psychoactive and neuroactive properties, was among the constituents, the observed effects should be attributed to the full-spectrum extract rather than DMT in isolation. Other compounds may have acted synergistically or antagonistically, influencing the final outcomes.
Lindvall and Bjorklund proposed the use of cell therapy as a strategy for treating neurodegenerative diseases, emphasizing the accessibility and suitability of somatic stem cells for such applications (21). Similarly, Hermann et al. demonstrated that stem cells are responsive to neural markers, reinforcing the therapeutic potential of neural stem cells, which possess the capacity for self-renewal and differentiation into neurons, astrocytes, and oligodendrocytes (22). The use of plant-derived compounds in medical treatments has gained increasing attention, and traditional medicine has long recognized Acacia species for their anti-nausea, analgesic, and anti-inflammatory effects (23). However, little research has explored their influence on stem cells. Acacia extracts have also demonstrated antidiabetic, anxiolytic, antioxidant, and anticancer properties. Some studies report that these extracts can enhance glucose uptake and induce apoptosis in breast cancer cells while inhibiting their growth (24).
Rajasekaran et al. showed that treatment of neuronal cells with rose essential oil increased neuronal cell numbers, indicating the potential for neurogenesis (25). Many natural plant extracts contain compounds that mimic endogenous neurotrophic factors, promoting neuronal survival and possibly stimulating neurogenesis. In the central nervous system, neurotrophic factors closely interact with the cholinergic system, supporting the survival and phenotypic expression of damaged cholinergic neurons (25).
Previous studies have demonstrated that DMT exerts neuroprotective effects against ischemic brain damage through sigma-1 receptor (S1R)-mediated mechanisms in murine models of middle cerebral artery occlusion (MCAO). Nardai et al. observed a significant reduction in infarct volume 24 hours post-MCAO, along with improved functional recovery of the impaired limb after 30 days of DMT treatment (26). These findings are consistent with other preclinical studies that have confirmed DMT’s neuroprotective role in ischemia-reperfusion injury, including those involving human-based models such as brain organoids (27). While earlier studies highlighted DMT’s action via S1R activation, the present study, due to the use of a complex extract, cannot isolate the specific contribution of DMT.
A proposed mechanism for the neuroprotective and neuroplasticity-promoting effects of psychedelics involves the upregulation of BDNF. The BDNF is a well-established mediator of neurogenesis, neurite outgrowth, dendritic branching, and spinogenesis — the formation of dendritic spines (28). These processes are vital for the maturation of the nervous system and are largely driven by dynamic cytoskeletal remodeling. The PC12 cell line, derived from rat adrenal pheochromocytoma, was first introduced by Greene and Tischler in 1976 (29). These cells produce and release catecholamines, including dopamine and norepinephrine, and are widely employed as a model for studying neuronal differentiation, neurotoxicity, and neurodegeneration (30). Due to their neurochemical properties and neuronal phenotype, PC12 cells represent a reliable model in neurobiological research.
In the present study, this cell line was used to evaluate the cytotoxic effects of Acacia extract. Our findings suggest that DMT, especially at low concentrations, may exert neurogenic effects on neuronal cells, potentially offering therapeutic value in conditions such as Alzheimer’s disease and depression. However, as the extract contained other compounds, including potentially toxic rubranins, some of the observed toxic effects may be attributed to the extract mixture rather than DMT alone. It is plausible that treatment with pure DMT might yield more favorable outcomes. Nevertheless, toxicity was only observed at higher concentrations and with prolonged exposure. Notably, no apoptosis was observed at concentrations of 0.4 µg/mL and 0.8 µg/mL, and ROS levels at 0.4 µg/mL after 48 hours did not show a significant increase.
5.1. Conclusions
This study demonstrates the promising neurogenic potential of Acacia extract, particularly at a low concentration of 0.4 µg/mL and following short-term exposure (24 hours). The increase in BDNF gene expression observed under these conditions highlights the possible role of Acacia-derived bioactive compounds, especially N,N-DMT, in promoting neurogenesis and supporting neuronal health. Given DMT’s previously documented neuroprotective effects via S1R activation, its presence in the Acacia extract likely contributes to the observed outcomes. However, due to the use of a whole-plant extract that includes other active and potentially toxic compounds, such as rubranins, the specific role of DMT could not be isolated. It is possible that the observed toxic effects at higher concentrations and longer exposure durations were influenced by components other than DMT. Importantly, at concentrations of 0.4 µg/mL and 0.8 µg/mL, no signs of apoptosis were detected, and ROS levels remained within non-toxic ranges after 48 hours at the lower dose. The results suggest that pure DMT may yield even more favorable outcomes with fewer cytotoxic effects, warranting further investigation. Additionally, this study emphasizes the therapeutic promise of exploring various species of Acacia, particularly those indigenous to southern Iran, for their neuroprotective and neurogenic potential. Overall, these findings contribute to the growing body of evidence supporting the use of plant-derived compounds in neuroregenerative medicine. They point to new avenues for the development of novel therapeutic agents aimed at treating cognitive impairments and neurodegenerative disorders such as Alzheimer’s disease and depression.