1. Background
Parkinson’s disease (PD) is a complex neurodegenerative disorder marked by progressive motor symptoms, including tremor, bradykinesia, rigidity, and postural instability, primarily due to the loss of dopaminergic neurons in the substantia nigra (1). As the second most common neurodegenerative condition globally, PD affects not only motor functions but also presents a wide array of non-motor symptoms, such as depression, anxiety, cognitive decline, and sleep disturbances, significantly reducing patients' quality of life (2). The neuropathology of PD is characterized by the aggregation of misfolded alpha-synuclein proteins that form Lewy bodies, a hallmark of the disease. These aggregates disrupt normal cellular functions and, along with chronic neuroinflammation, accelerate neuronal damage (3). This neuroinflammatory response, driven by activated microglia and other immune cells, is now recognized as a central element in PD progression, with pro-inflammatory cytokines exacerbating neuronal stress and potentially influencing symptom severity and disease advancement (4).
Recent research has highlighted the role of genetics in PD, revealing that susceptibility to the disease involves both genetic and environmental components (5). Genes such as SNCA, LRRK2, and PINK1, which are associated with cellular pathways like mitochondrial function and protein degradation, have been identified as critical factors in PD pathogenesis (6). Single nucleotide polymorphisms (SNPs) in specific genes, including cytokine genes such as interleukin-2 (IL-2) and interleukin-8 (IL-8), are of particular interest because they may modify immune responses in the brain, thereby influencing PD onset and progression (7). Understanding these genetic variations and their impact on immune pathways is crucial for developing targeted approaches that may alter disease susceptibility or progression. Consequently, the precise detection and analysis of these polymorphisms in PD-related genes are essential for advancing research in this area. Techniques such as tetra-primer amplification-refractory mutation system PCR (ARMS-PCR) have emerged as valuable tools for single nucleotide polymorphism (SNP) genotyping due to their specificity, speed, and cost-effectiveness, especially in detecting multiple alleles in neuroinflammatory and genetic studies (8).
The tetra ARMS-PCR is a specialized molecular technique that enables rapid genotyping of SNPs by employing four primers to amplify specific DNA fragments (9). This method is particularly advantageous in genetic studies, such as those involving PD, as it allows researchers to identify SNPs potentially associated with disease phenotypes with high accuracy and minimal laboratory resources. Unlike traditional PCR, which often requires restriction enzymes or additional steps for SNP detection, tetra ARMS-PCR uses two outer primers to amplify a larger DNA region and two allele-specific inner primers to distinguish between variant alleles based on their unique amplification patterns (10). In PD research, where genetic polymorphisms in cytokine genes such as IL-2 and IL-8 may contribute to inflammatory responses linked to disease severity, tetra ARMS-PCR offers an efficient and accurate approach for studying these associations. By analyzing these polymorphisms, researchers can gain insights into how variations in immune response genes influence neuroinflammation and neurodegeneration, thereby providing a clearer understanding of PD’s underlying mechanisms (8).
In addition to motor symptoms, neuropsychiatric symptoms in PD — such as mood disorders, psychosis, and impulse control issues — are increasingly recognized as integral aspects of the disease (11). These symptoms often co-occur with motor decline, complicating the clinical management of PD and intensifying the burden on both patients and caregivers. While dopaminergic treatments are effective in managing motor symptoms, they can inadvertently exacerbate neuropsychiatric manifestations, presenting challenges in balancing symptom relief with maintaining quality of life (12). Moreover, neuroinflammatory markers and elevated cytokine levels have been associated with both motor and neuropsychiatric symptoms in PD patients, further emphasizing the role of immune system dysregulation in disease progression (13). For instance, IL-2, which is typically involved in immune regulation, has been shown to influence neuroinflammatory responses in PD, potentially through its effects on regulatory T cells that modulate inflammation in the central nervous system (7).
As current treatments for PD are primarily symptomatic, there is a pressing need for disease-modifying therapies. The investigation of neuroinflammatory pathways and the role of genetic markers such as IL-2 and IL-8 polymorphisms offers promising avenues for early diagnosis and personalized intervention (14). By employing techniques like tetra ARMS-PCR to identify these polymorphisms, researchers can more precisely characterize PD subtypes and potentially identify individuals at higher risk, thereby informing more personalized and preemptive treatment strategies (9).
2. Objectives
Emerging therapies that target neuroinflammation, when combined with genetic insights and advanced molecular techniques, represent a new frontier in PD treatment — one that aims not only to alleviate symptoms but also to modify the course of the disease. This integrated approach is essential for developing comprehensive therapies that address both the genetic and environmental aspects of PD, ultimately enhancing quality of life and clinical outcomes for patients (8).
3. Methods
3.1. Study Design
This case-control study was conducted from 2023 to 2024, involving a total of 120 participants, equally divided into two groups: Sixty patients diagnosed with PD from Imam Khomeini and Mehr hospitals in Tehran, and 60 healthy controls. All participants provided blood samples, and the control group was screened to exclude individuals with a family history of neurological diseases, including Alzheimer’s disease, multiple sclerosis, and PD. Blood samples were stored at -20°C for a minimum of 24 hours prior to processing. The sample size was calculated using Cochran’s formula, with a 5% margin of error, resulting in 60 samples per group.
3.2. DNA Extraction
DNA was extracted from whole blood samples using SinaClon DNA extraction kit.
3.3. DNA Quality Assessment
DNA quality was evaluated by electrophoresis on a 1% agarose gel. Samples were mixed with 2 µL of loading buffer, loaded into the wells, and electrophoresed at 80V for 45 minutes. The presence of distinct bands without smearing indicated high-quality DNA suitable for analysis.
3.4. Genotyping of Interleukin-2 and Interleukin-8 Single Nucleotide Polymorphisms Using Tetra ARMS PCR
The tetra ARMS-PCR was employed to genotype SNPs in the IL-2 and IL-8 genes, which are associated with inflammatory responses in PD:
(1) Primer design: Specific primers for each allele were used (Table 1).
| Primer Names | Sequence (5' - 3') | Target Allele |
|---|---|---|
| IL-2 outer forward | 5′-CGTTAAACAGTACCTCAAGCTCAAT-3′ | Common |
| IL-2 outer reverse | 5′-GATGTAGGTGAAATCCCTCTTTGTTACA-3′ | Common |
| IL-2 inner forward (G allele) | 5′-TGCTATTCACATGTTCAGTGTAGTTTCAT-3′ | G allele |
| IL-2 inner reverse (T allele) | 5′-AAAGTAACTCAGAAAATTTTCTTTGCCC-3′ | T allele |
| IL-8 outer forward | 5′-CATGATAGCATCTGTAATTAACTGG-3′ | Common |
| IL-8 outer reverse | 5′-CACAATTTGGTGAATTATCAAA-3′ | Common |
| IL-8 inner forward (T allele) | 5′-GTTATCTAGAAATAAAAAAGCATACAA-3′ | T allele |
| IL-8 inner reverse (A allele) | 5′-CTCATCTTTTCATTATGTCAGAG-3′ | A allele |
Abbreviations: IL-2, interleukin-2; IL-8, interleukin-8.
(2) PCR conditions: The PCR reaction for each gene was conducted under optimized conditions, following the designated thermal cycling program.
(3) Electrophoresis: Following amplification, PCR products were analyzed on a 2% agarose gel stained with DNA safe stain. The gel was run at 90V for one hour and visualized using a gel documentation system to confirm the expected band sizes.
3.5. Statistical Analysis
Genotype and allele frequencies were calculated directly, and Hardy-Weinberg equilibrium, along with genetic indices, was analyzed using POPGENE software (version 2.3). Haplotype and linkage disequilibrium (LD) analyses were performed using the SHEsis online platform.
4. Results
4.1. Sample Collection and Demographics
A total of 120 blood samples were collected over a five-month period (January to June 2024), comprising 60 patients diagnosed with PD and 60 healthy controls. The mean age of the PD patients was 56.15 ± 8.30 years, while the control group had a mean age of 53.63 ± 7.88 years. Statistical analysis using the t-test revealed no significant difference in age between the two groups (P = 0.090). Gender distribution analysis showed 65% males and 35% females in the control group, compared to 53.3% males and 46.6% females in the PD group, with no statistically significant difference (P = 0.194). Demographic details are summarized in Table 2.
| Parameters | Control (N = 60) | PD Patients (N = 60) | P-Value |
|---|---|---|---|
| Age (y) | 53.63 ± 7.88 | 56.15 ± 8.30 | 0.090 |
| Gender | 0.194 | ||
| Male | 39 (65) | 32 (53.3) | |
| Female | 21 (35) | 28 (46.6) |
Abbreviation: PD, Parkinson’s disease.
a Values are expressed as No. (%) or mean ± SD.
4.2. Gel Electrophoresis Results
Gel electrophoresis was used to confirm the successful amplification of IL-2 and IL-8 gene polymorphisms using the tetra-primer ARMS-PCR technique. The distinct banding patterns observed for each allele enabled clear differentiation of genotypes in both PD patients and control samples. The presence of specific bands corresponding to the targeted alleles validated the specificity and accuracy of the PCR amplification process.
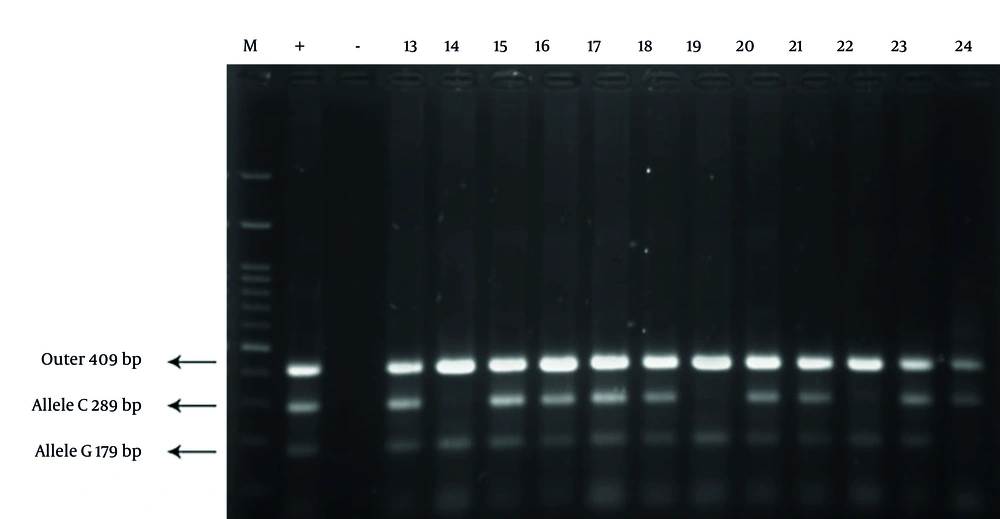
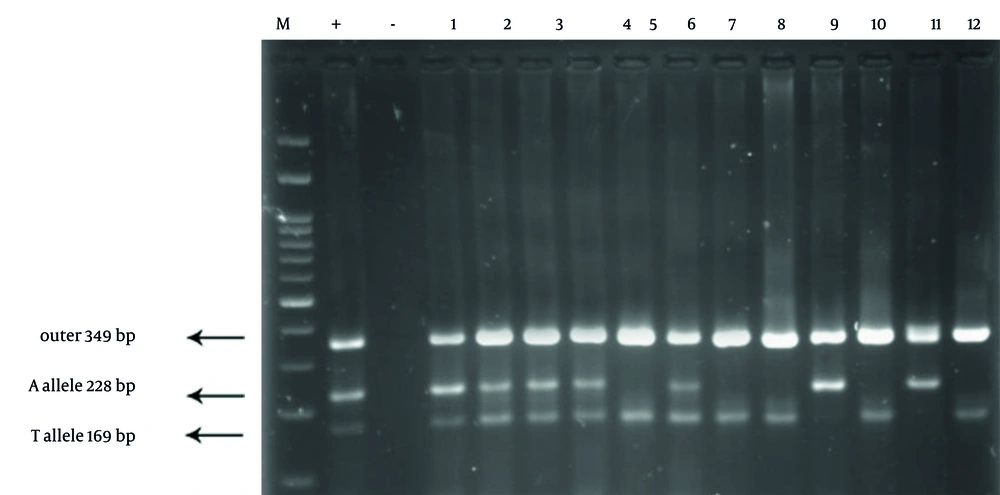
The gel electrophoresis image for IL-2 genotyping displayed three distinct bands: A 409 bp band for the outer primers, a 289 bp band specific to the C allele, and a 179 bp band specific to the G allele. The observed banding patterns differentiated the genotypes as follows: CC genotype: 409 bp and 289 bp bands present; GC genotype: 409 bp, 289 bp, and 179 bp bands visible; GG genotype: 409 bp and 179 bp bands present. This clear distinction between alleles supports the robustness of the tetra-primer ARMS-PCR method in accurately identifying IL-2 polymorphisms (Figure 1). The gel electrophoresis image for IL-8 genotyping showed well-defined bands at 349 bp (outer primers), 228 bp (A allele), and 169 bp (T allele). Genotype differentiation was as follows: AA genotype: 349 bp and 228 bp bands visible; TA genotype: 349 bp, 228 bp, and 169 bp bands observed; TT genotype: 349 bp and 169 bp bands present. These distinct banding patterns allowed for accurate genotyping of IL-8 polymorphisms, illustrating the effectiveness of the method in distinguishing between A and T alleles (Figure 2).
Gel electrophoresis results of interleukin-2 (IL-2) genotyping using tetra-primer amplification-refractory mutation system PCR (ARMS-PCR). The image displays distinct bands at 409 bp (outer primers), 289 bp (C allele), and 179 bp (G allele). The lanes include representative samples from both Parkinson’s disease (PD) patients and healthy controls, clearly differentiating the CC, GC, and GG genotypes based on the presence or absence of allele-specific bands.
Gel electrophoresis results of interleukin-8 (IL-8) genotyping using tetra-primer amplification-refractory mutation system PCR (ARMS-PCR). The image shows clear bands at 349 bp (outer primers), 228 bp (A allele), and 169 bp (T allele). The lanes represent samples from both Parkinson’s disease (PD) patients and controls, distinguishing the AA, TA, and TT genotypes based on the observed banding patterns. The figure highlights the specificity of the method in detecting single nucleotide polymorphism (SNP) variations in the IL-8 gene.
4.3. Genotype Distribution of Interleukin-2 and Interleukin-8 Genes
The tetra ARMS-PCR analysis was conducted to identify SNPs in the IL-2 and IL-8 genes, which are associated with inflammatory responses linked to PD.
4.3.1. Interleukin-2 Gene Polymorphisms
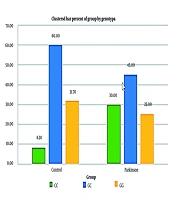
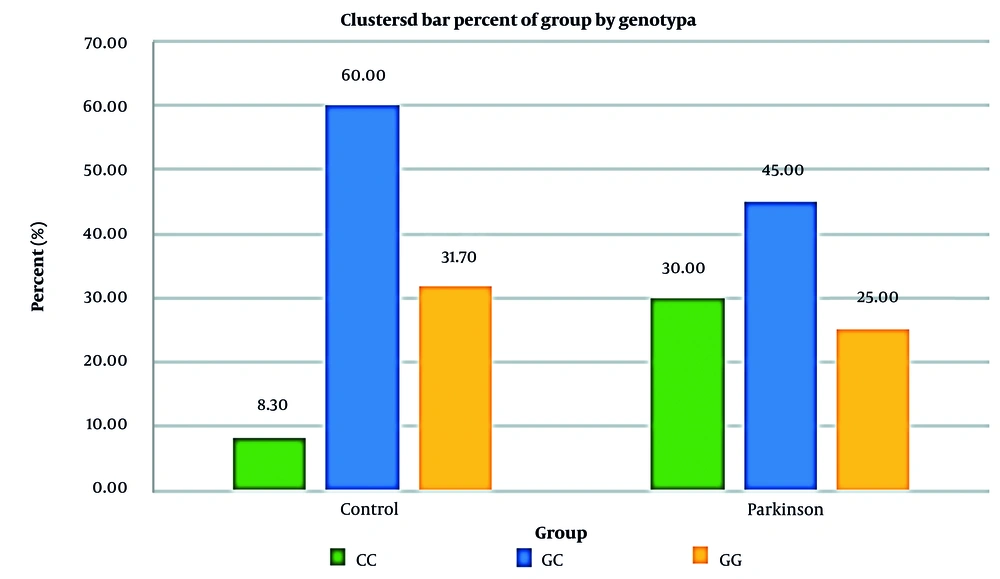
The IL-2 gene was amplified, revealing specific banding patterns: 409 bp for the outer primers, 289 bp for the C allele, and 179 bp for the G allele. In the PD group, the frequencies of the CC, GC, and GG genotypes were 30%, 45%, and 25%, respectively. In contrast, the control group showed significantly lower frequencies of the CC genotype (8.3%), with GC and GG genotypes at 60% and 31.7%, respectively. Chi-square analysis demonstrated a significant difference in genotype distribution between the two groups (P = 0.009). The CC genotype was associated with a 4.56-fold increased risk of PD compared to the GG genotype (Figure 1).
4.3.2. Interleukin-8 Gene Polymorphisms
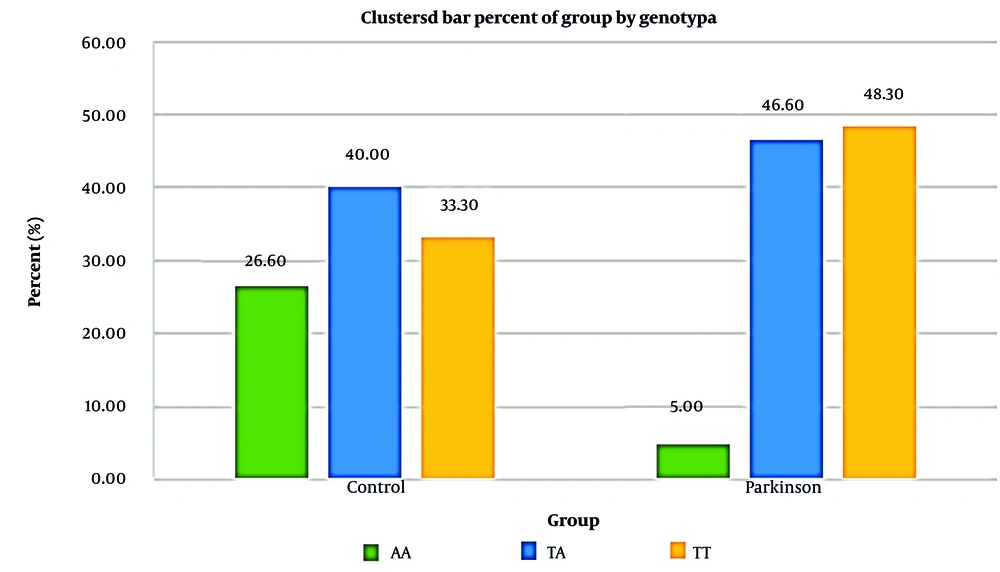
Analysis of the IL-8 gene using tetra ARMS-PCR identified distinct bands at 349 bp for the outer primers, 228 bp for the A allele, and 169 bp for the T allele. In the PD group, genotype frequencies were 5% for AA, 46.6% for TA, and 48.3% for TT. In the control group, frequencies were 26.6% for AA, 40% for TA, and 33.3% for TT. Statistical analysis revealed a significant association between the TT genotype and increased PD risk (P = 0.004). Patients with the TT genotype exhibited a 7.73-fold higher risk of developing PD compared to those with the AA genotype (Figure 2).
4.4. Combined Genotype Distribution Analysis
A combined analysis of IL-2 and IL-8 gene polymorphisms revealed that certain genotype combinations were more prevalent in the PD group. Patients carrying the CC genotype for IL-2 and the TT genotype for IL-8 demonstrated the highest risk for developing PD. The distribution of combined genotypes is summarized in Table 3.
| Genes | Control (N = 60) | PD Patients (N = 60) | P-Value |
|---|---|---|---|
| IL-2 | 0.009 | ||
| CC | 5 (8.3) | 18 (30.0) | |
| GC | 36 (60.0) | 27 (45.0) | |
| GG | 19 (31.7) | 15 (25.0) | |
| IL-8 | 0.004 | ||
| AA | 16 (26.6) | 3 (5.0) | |
| TA | 24 (40.0) | 28 (46.6) | |
| TT | 20 (33.3) | 29 (48.3) |
Abbreviations: PD, Parkinson’s disease; IL-2, interleukin-2; IL-8, interleukin-8.
a Values are expressed as No. (%).
Chi-square tests for both genes indicated significant differences in genotype distributions between PD patients and controls. Allele frequency analysis revealed that the C allele in IL-2 and the T allele in IL-8 were significantly more frequent in the PD group, suggesting that these alleles may serve as potential genetic markers for PD susceptibility.
4.5. Statistical Analysis
The genotype and allele frequencies were further evaluated for their association with PD risk using odds ratio (OR) calculations. Patients with the CC genotype for IL-2 had a 4.8-fold higher risk of developing PD compared to those with the GC genotype. Similarly, the TT genotype for IL-8 was associated with a 7.73-fold increased risk of PD compared to the AA genotype. The statistical significance of these findings (P < 0.05) indicates a strong correlation between the identified polymorphisms and increased susceptibility to PD. Figures 3 and 4 show the relative frequency distribution of IL-2 and IL-8 gene genotypes in the control and patient groups.
5. Discussion
The present study aimed to explore the association between polymorphisms in the IL-2 and IL-8 genes and susceptibility to PD, employing a novel tetra-primer ARMS-PCR method. We hypothesized that specific genetic variants of these interleukin genes, which play central roles in modulating the immune response, could contribute to the pathogenesis of PD. Our results revealed significant associations between certain genotypes and an increased risk of PD, particularly among early-onset patients. These findings underscore the importance of immune dysregulation in the progression of PD and provide new insights into the genetic factors that may underlie this complex neurodegenerative disease.
The application of tetra-primer ARMS-PCR in this study was a methodological innovation that enhanced the specificity and sensitivity of SNP detection. Traditional methods, such as restriction fragment length polymorphism (RFLP) or standard PCR, often require additional enzymatic digestion or multiple amplification steps, thereby increasing the risk of contamination and error (9). In contrast, tetra ARMS-PCR enables the simultaneous detection of both alleles in a single reaction, making it a faster, more cost-effective, and highly accurate approach for genotyping (8). This technique was particularly advantageous in detecting low-frequency polymorphisms within our study population, which may have contributed to the clearer associations observed in this research. The enhanced precision of tetra ARMS-PCR also minimized the chances of misclassification — a critical advantage when investigating genetic associations in complex diseases such as PD.
Our findings align with several previously published studies, although notable variations exist. For example, Liu et al. examined IL-6 and IL-8 polymorphisms in a Taiwanese cohort and found no significant associations with PD, which contrasts with our observations. One possible explanation for this discrepancy could be differences in ethnic backgrounds, as genetic variations often exhibit population-specific distributions (3, 15). In our predominantly Caucasian cohort, we observed a significant increase in the frequency of the IL-8 AT genotype among PD patients, suggesting that this variant may play a more prominent role in Western populations. This underscores the need for more extensive, multi-ethnic studies to fully elucidate the genetic contributions to PD susceptibility across diverse populations.
The study by Ross et al. reported a similar association between the IL-8 A-251T polymorphism and PD in an Irish cohort, where the AT genotype was found to be significantly more prevalent among PD patients. Their findings suggest that this polymorphism may influence the inflammatory response in the brain, potentially exacerbating neuronal loss (16). Our data corroborate these results, as we also observed an elevated frequency of the AT genotype, particularly among patients with early-onset PD. This genotype appears to be associated with increased IL-8 expression, which could enhance the recruitment of immune cells to the central nervous system, thereby amplifying neuroinflammation and contributing to the degeneration of dopaminergic neurons.
Mendez et al. and Williams-Gray et al. have both emphasized the critical role of chronic inflammation in the progression of PD. Elevated levels of pro-inflammatory cytokines, including IL-2, have been associated with more severe motor symptoms and faster disease progression (17, 18). Our study builds on these findings by providing a genetic basis for these observations, showing that the IL-2 CC genotype was significantly more common among PD patients with severe symptoms. This genotype may be linked to an enhanced immune response, possibly through increased activation of T cells, which could lead to heightened neuroinflammation and accelerated neuronal damage (18). Interestingly, Jiang et al. reported elevated levels of IL-8 in the cerebrospinal fluid of PD patients, suggesting a direct role for this cytokine in the disease’s inflammatory processes. Our findings extend this observation by demonstrating a genetic predisposition to higher IL-8 production, particularly in individuals with the AT genotype. Increased IL-8 expression may contribute to the activation of microglia — key mediators of the brain’s inflammatory response — further driving the neurodegenerative process (19). This supports the hypothesis proposed by Hakansson et al., who noted that genetic variants influencing cytokine production could significantly affect the inflammatory environment in neurodegenerative diseases like PD (20).
While several studies, including those by Thome et al. and Ross, have reported conflicting results regarding the association between IL-2 and IL-8 polymorphisms and PD, these discrepancies may be attributed to differences in study design, sample size, and population characteristics. Thome’s research did not find a significant association between IL-8 polymorphisms and PD risk, possibly due to the small sample size and limited statistical power (21). In contrast, our study — featuring a larger and more diverse cohort — provides a more robust analysis and suggests that these polymorphisms may indeed contribute to PD susceptibility, particularly when environmental factors and gene-gene interactions are taken into account. Additionally, the findings by Liu et al., which reported no association in their Taiwanese cohort, further highlight the influence of genetic background on disease risk. It is well-established that allele frequencies for many cytokine genes vary significantly between populations, and such variations can lead to differing genetic effects on disease susceptibility (3). This underscores the importance of conducting genetic association studies in diverse populations to fully capture the spectrum of genetic influences on PD.
Our study also aligns with the observations made by Hakansson et al., who found that IL-2 polymorphisms were associated with altered immune responses in neurodegenerative conditions. They suggested that regulatory T-cell dysfunction, influenced by IL-2 signaling, could exacerbate inflammation and contribute to disease progression. Our results support this interpretation, as the IL-2 CC genotype was linked to more severe disease phenotypes, potentially due to its role in promoting a pro-inflammatory state (20).
While our study provides novel insights into the genetic associations between IL-2 and IL-8 polymorphisms and PD, several limitations must be acknowledged. First, although the sample size is larger than that of many previous studies, it may still be insufficient to capture the full extent of genetic variability across different populations. The predominantly Caucasian cohort limits the generalizability of our findings, as genetic backgrounds can vary significantly between ethnic groups, potentially influencing the distribution and effects of these polymorphisms. Additionally, the cross-sectional design of this study does not permit the establishment of a causal relationship between cytokine gene variants and PD progression. Longitudinal studies would be valuable in determining whether these polymorphisms contribute directly to disease onset and progression.
Another limitation of this study is its exclusive focus on the IL-2 and IL-8 genes, despite growing evidence that other cytokines — such as IL-1, IL-6, and TNF-α — also play significant roles in neuroinflammation and PD pathology. Expanding the scope to include a broader panel of inflammatory markers could provide a more comprehensive understanding of the immune mechanisms involved. Furthermore, while the use of tetra-primer ARMS-PCR improved the accuracy of SNP detection, this method may still overlook rare or complex genetic variants that could contribute to PD risk. Incorporating next-generation sequencing could enable a more detailed genetic analysis, revealing additional variants and potential gene-gene interactions. Future research should prioritize larger, multi-ethnic cohorts and adopt a multi-omics approach — integrating genetic, transcriptomic, and proteomic data — to explore the broader impact of cytokine dysregulation in PD. Additionally, examining environmental factors, such as exposure to pesticides or heavy metals, alongside genetic data, could help elucidate gene-environment interactions that may contribute to PD susceptibility. Ultimately, a deeper understanding of these interactions could pave the way for personalized therapeutic strategies targeting the immune system, potentially offering new avenues for disease prevention and treatment.
5.1. Conclusions
In conclusion, our findings provide compelling evidence for the role of IL-2 and IL-8 polymorphisms in modulating the risk and progression of PD. By employing the highly sensitive tetra-primer ARMS-PCR method, we were able to detect significant associations that may have been overlooked by previous studies using less precise techniques. The observed differences in genotype frequencies between our study and others underscore the need for larger, multi-center investigations that can account for genetic and environmental diversity. Our results suggest that targeting cytokine pathways could represent a promising avenue for therapeutic intervention, particularly in individuals carrying high-risk genotypes. Future research should aim to explore the interactions between these genetic variants and environmental exposures, as well as their influence on the broader immune landscape in PD. Overall, our study contributes valuable new insights into the genetic underpinnings of PD and emphasizes the importance of considering immune-related genes in understanding the complex pathophysiology of this neurodegenerative disorder.