1. Background
Epilepsy is the most common neurological disorder after stroke and Alzheimer disease; approximately one percent of world population is suffering from one type of epilepsy disorder (1). Epileptic seizures are generally divided into two categories: focal and generalized. Temporal lobe epilepsy is the most common focal seizure subtype in adults which results from repeated-release foci in medial temporal structures such as hippocampus and amygdale and is associated with hippocampal degeneration and related functional changes (2). Epilepsy is associated with a specific structural lesion in the hippocampus, which may be surgically resected in medically intractable cases. Investigation of temporal lobe epilepsy is stimulated, in part, by the involvement of the hippocampus which has a simple organization compared with other parts of the cerebral cortex and plays a role in learning and memory (2). Insights gained from studying temporal lobe epilepsy may be applied to other types of epilepsy and help reveal mechanisms of temporal lobe function. Epilepsy is usually treated symptomatically. Antiepileptic medications will reduce the epileptic seizures, meanwhile, in only 40% of patients epileptic seizures will be disappeared (3).
In recent years, naturally-driven compounds have been increasingly used in the protective treatment of the central nervous system disorders. An anti-convulsive effect of Vit E has been previously reported in the seizure induced by pilocarpine, penthyl-tetrazole, and penicillin (4-6).
2. Objectives
Considering the fact that kainic acid-induced epilepsy in the rat pathophysiologically can make a replica of that of temporal lobe epilepsy in humans (which is of considerable clinical significance) (7), we investigated the anti-convulsive effects of vit E in kainic acid-induced epilepsy in the rat.
3. Materials and Methods
In this experimental study, 40 Wister rats, weighing 250 - 300 g were used. All rats were kept at 21 - 23°C and had free access to standard food and water. Rats were randomly assigned to five groups (sham, sham + Vit E, epileptic, positive control (epileptic + Valproic acid treatment), and epileptic + Vit E). Epilepsy was induced by stereotactic surgery as follows: Rats were first anesthetized by a mixture of ketamin (100 mg/ kg) and xylazine (20 mg/kg). Kainic acid was dissolved in normal saline (sigma, USA) and was injected with a total dose of 4 microgram in each rat in the CA3 area in the right hippocampus (stereotactic coordinates: antero-posterior: 4.3 mm, lateral: 4.2 mm to bregma and ventral: 4.2 - 4.4 mm under the skull surface). Injections were administered using Hamilton syringe with a total volume of 5 μL. Sham-treated rats only received the same volume of normal saline. Vit E (100 mg/kg) was injected intraperitoneally on a daily basis for the week preceding the surgery. Valproic acid was injected with the same standard and with a dose of 200 mg/kg. In the immediate 24 hours following the surgery, convulsive behavior was videotaped and graded according to the Racine’s criteria (8). Convulsive behavior was again registered after two weeks. Summary of Racine’s criteria is as follows:
No activity
Mounting, blinking or mild clonus
Head shaking or multiple clonus in head
Myclonic jerks in anterior limbs
Myoclonic convulsion in anterior limbs and standing on feet’s
Generalized clonic seizure and loss of balance
3.1. Statistical Analysis
Results of all measurements were reported as means ± SEM. To compare different experimental groups, one–way ANOVA and Tukey Test were performed. Differences between different statistical groups were considered statistically significant at P< 0.05.
4. Results
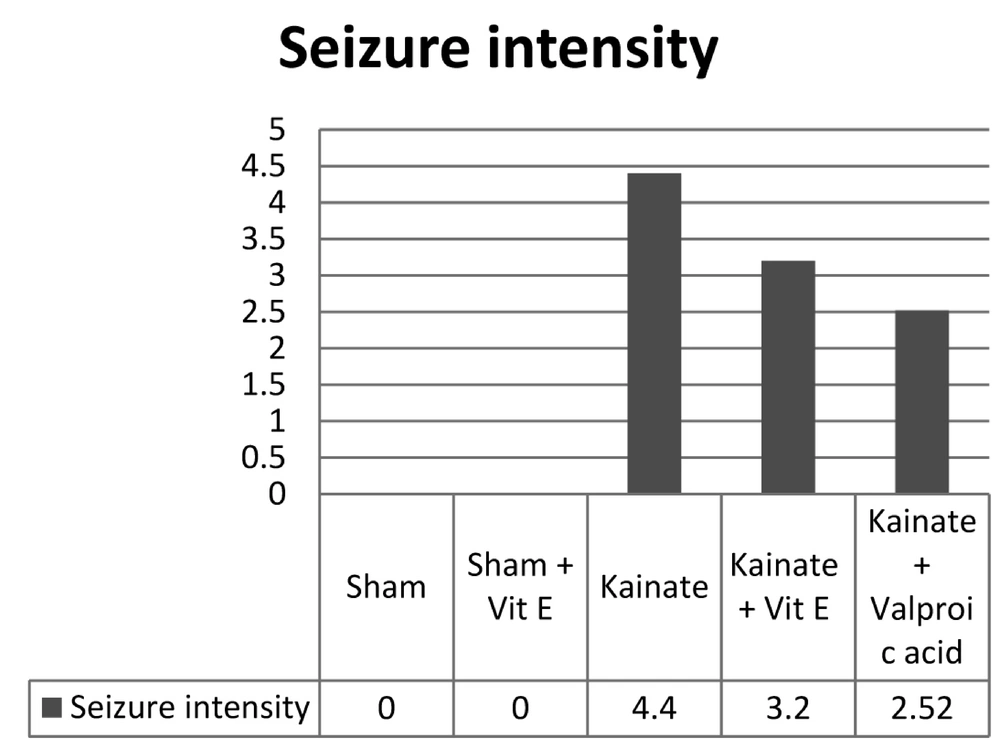
Figure 1 shows the quantitative evaluation of convulsive behavior of animals based on Racine’s classification (graded as 0 - 5). In control groups (sham-surgery and sham + Vit E pre-treatment) no convulsive behavior was observed in animals. According to the result of tukey test, in the kainic acid-induced seizure group, a marked and significant convulsive behavior was observed and pre-treatment with Vit E significantly reduced the convulsive behavior of animals (P < 0.05). Meanwhile, pre-treatment with valproic acid induced more pronounced decrease in the convulsive behavior of animals as compared with Vit E-pre-treatment group (P < 0.01).
5. Discussion
Results of the current study showed that Vit E administration decreases the intensity of convulsive attacks in rat. Temporal lobe epilepsy is a focal type of seizure that is manifested by repeated convulsive attacks (1). Previous studies have clearly shown the anti-convulsive effects of Vit E in pilocarpine-induced absent seizure in rats (9). Results of these studies show that Vit E reduces the seizure intensity and frequency by decreasing oxidative stress and lipid peroxidation in the seizure loci of brain. Likewise, Naziroglu and colleagues have shown that Vit E increases the total anti-oxidant capacity of brain (5). The same mechanism is also proposed for penthylenetetrazol and penicillin-induced seizure (4, 6). Autophagy is a cellular phenomenon that is increased in epilepsy in certain parts of brain and Vit E can decrease the autophagy and neuronal death (4). Vit E could also have exerted its anti- convulsive effect by protection against cellular injury induced by kainic acid in hippocampal neurons with a decrease in mossy fiber sprouting and subsequent reduction of seizure intensity in following days. Such a neuroprotective effect has been previously shown in other studies (10, 11). Additionally, anti-oxidants such as Vit E can prevent the cytochrome oxidase dysfunction and decrease high energy phosphate compounds and nitric oxide in different brain regions especially in hippocampus which leads to decreased severity of neuronal damage resulting from the kainic acid injection into the brain. This results in decreased alterations in the neuronal plasticity in hippocampus, leading to decreased seizure frequency and intensity (12). Meanwhile, since oxidative stress is one of the main responsible mechanisms for cell death induced by seizures in epilepsy including temporal lobe epilepsy, antioxidant compounds like vitamin E could have neuroprotective effects due to their ability to inhibit free radical production which in turn it could exert antiepileptic effect through inhibition of free radical production (2, 10). These results highlighted the promising therapeutic potential of vitamin E as an effective treatment for neurodegenerative diseases and epileptic condition. Pre-treatment with Vit E leads to decreased seizure intensity in kainic acid-induced epilepsy in rat.