1. Background
Von Heller first described Carotid Body Tumors (CBTs) or chemodectomas in 1743 (1). They originate from Type 1 chief cells of the carotid body and affect both sexes in the fourth and fifth decades of life. CBTs could be transmitted familially; (5 - 10%) in some cases they tend to be multi-centric, or they can be sporadically found in association with other paragangliomas. It is a slow-growing neoplasia, locally aggressive, and it can spread by metastasis to regional lymph nodes and the surrounding anatomical structures (2). For the first time in 1880, Reigner attempted to resect a CBT, but the patient did not survive. Scudder performed the first successful removal of a CBT in 1903. Once surgically removed, CBT can recur in a small percentage of patients (2). Shamblin described three anatomical groups for classification of CBTs, considering the tumor size and its vascular attachments (3).
Patients with CBTs usually present a gradually-enlarging nontender lateral neck mass. This mass is usually mobile in the horizontal direction (right to left/left to right), but fixed in vertical direction. They are usually benign and nonfunctional; their differential diagnosis includes metastatic lymph nodes, carotid artery aneurysm, salivary gland tumor, branchial cleft cyst, and neurogenic or thyroid tumors. When such a lesion is suspected, a noninvasive color Doppler flow ultrasonography enables the clinician to arrive at a definite diagnosis. Typically, digital subtraction angiography shows a well-defined, hypervascular tumor, situated at the common carotid arterial bifurcation of a carotid body tum, that widens the bifurcation. Computed tomography scanning is appropriate to delineate the relation of the tumor to the adherent structures, while magnetic resonance tomography demonstrates the relation of the tumor to the adjacent structures. Selective embolization is advised in large tumors for safe surgical removal with less bleeding. Prompt surgery is the treatment of choice and recommended in order to minimize the major risks (4). Different data are introduced in the literature of different countries. Even in Iran, a few studies have been conducted to show the distribution and features of this tumor (5, 6).
2. Objectives
Regarding the low incidence of this tumor, a remarkable number of patients in Shohadaye Tajrish hospital, and little published data from Iran, this study was performed to define the demographic features as well as clinical characteristics of Iranian patients with CBT during a 10-year period in a referral hospital.
3. Material and Methods
This was a retrospective study in which the medical records of patients diagnosed with CBT during the past ten years (from April 1st 2002 to April 1st 2012) were reviewed and the relevant data were collected and analyzed. The study was conducted at Shohadaye Tajrish vascular surgery referral center, Tehran, Iran. After the treatment, patients were followed up at the hospital clinic for a nonlimited duration and each visit was recorded in the patient’s medical file. In this study, all patients diagnosed with CBTs were enrolled, but patients with incomplete records were excluded.
Patients with an indexed final diagnosis of CBT were included. For the purpose of data extraction, a structured data collection instrument was used. The instrument was designed in six categories: demographic data, living conditions and predisposing factors, symptoms upon presentation, imaging results, treatment, complications, and follow up. The demographic data section included the patient’s age, sex, ethnicity and occupation. In the second section we tried to pinpoint any predisposing factors. This included the living place altitude, family history of endocrine disorders, or positive history of malignancy. Surgery reports were used as references for the tumor size and the extent of the local invasion. The follow-up information was extracted from the patient’s medical file at the hospital clinic.
Follow-ups were done by a checklist comprised of postoperative complications including dysphasia, hoarseness, slow-growing mass on the same side or the other side of the neck, and new-onset mass in other family members. The study was approved by the hospital ethics committee. The data were analyzed by the chi-squared test and T-test when applicable, using SPSS version 17.0. P-values ≤ 0.05 were considered statistically significant.
3.1. Operative Procedure
A similar surgical technique (periadventitial CBT resection) was performed for all patients. With positioning the patient's neck rotated to the opposite side, the standard anterolateral cervical incision along the anterior border of sternocleidomastoid would be obtained. The following steps would be carried out for the carotid body tumor excision: performing the control of common, internal, and external carotid arteries as well as vagal and hypoglossal nerves exploration, ultimately obtaining of an inferior margin of the tumor plane would be possible. Precise surgical care limits blood loss. The surgical techniques used for unilateral and bilateral CBTs were similar.
4. Results
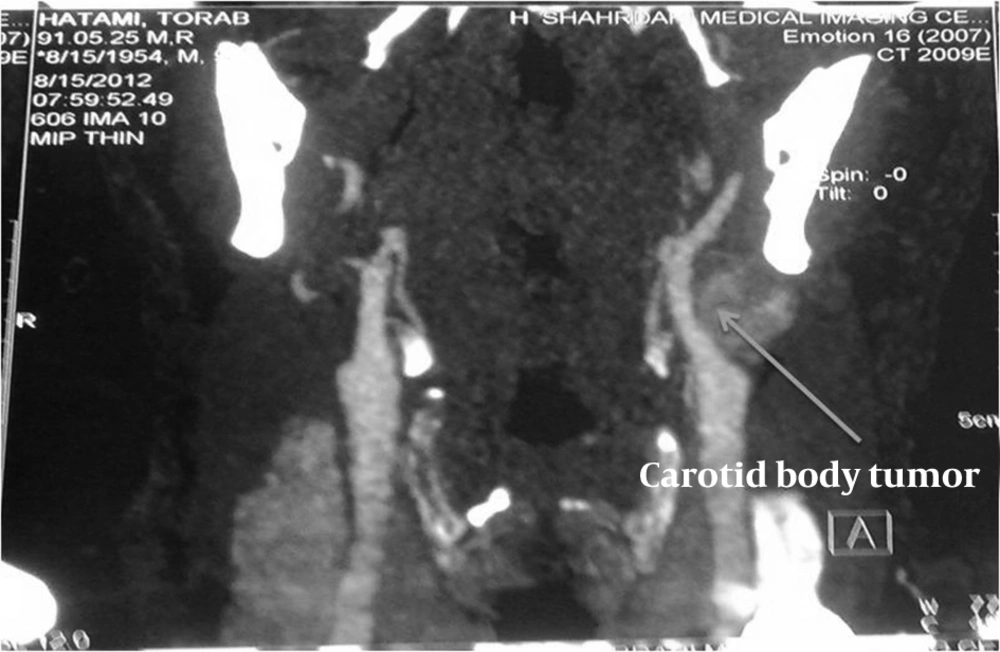
Overall, 48 patients with 51 CBTs were included in the study. We found an overwhelming predominance of women (18 men, aged 31 - 45 years and 30 women, aging 51 - 61 years) with a female-to-male ratio of 1.6:1. The mean age of included patients was 44 years, ranging from 18 to 75. Thirty patients lived at a place with an altitude of higher than 1500 m (58.8%), and 21 lived at equal or less than 1500 m (41.17%). In this study, three and five patients had diabetes mellitus and hypertension, respectively. Eighteen (35.2%) patients had a history of smoking. Unilateral or bilateral painless progressive mass in the neck with normal vital signs was the major complaint of all the cases (100%). The study was conducted at Shohadaye Tajrish medical center which is a major vascular surgery referral center in Tehran. Six (11.7%) patients elucidated additional symptoms of ipsilateral cranial nerve palsy either in the 10th or 12th cranial nerve. Neuroendocrine symptoms were not obvious at the presentation. Twenty-nine (56.8%) CBTs were pulsatile, and 23 (45.09%) had bruit on auscultation. Considering the bruit, only 11 (21.5%) patients with CBTs had pulsatile mass in the physical examination. Twenty three tumors were located on the right side and 22 on the left; 3 (6%) patients had bilateral chemodectomas. Duration of symptoms varied widely between 3 months and 13 years with a mean of 2.5 years. No patient had a history of malignancy or a familial history of carotid body tumors. Of all the patients, 11 (23%) were highlanders. The most common helpful imaging technique for obtaining diagnosis was the color Doppler sonography performed in 45 (84.3%) patients. Computed tomography (Figure 1), angiography, and magnetic resonance imaging (MRI) were performed in 26 (50.9%), 11 (21.5%), and 3 (5.8%) patients, respectively. CT +angiography, Doppler + CT, Doppler + CT + angiography, and Doppler + angiography were obtained in eight, 21, four, and eight patients respectively.
All the patients were treated by surgical resection while three received irradiation therapy postoperatively due to their nonresectable masses. Tumoral involvement of the sternocleidomastoid muscle and massive invasions to common carotid artery (CCA), internal carotid artery (ICA), and external carotid artery (ECA) were reported only in one patient, therefore the ICA could not be safely dissected of the surrounding tissue and treatment with irradiation was mandated. In another patient there was intraoperative injury to the ICA, which was repaired after shunt placement and primary closure. In a third patient, surgery was performed after angioembolization of the chemodectoma. Ligation of the carotid artery was never necessary. No perioperative deaths occurred. The mean tumor size was 3.2 * 2.7 * 1.8 cm. General pathologic examinations confirmed the chemodectomas without malignancy. Postoperative follow-ups ranged from 9 months to 9 years with a mean of 38 months. Three cases of cranial nerve palsy (X and XII cranial nerves) persisted after surgery mainly due to the inevitable resection of an invasive neoplasm. There was no evidence of recurrency over the mean follow-up period of 38 months and all the resected tumors remained disease-free and asymptomatic.
5. Discussion
The incidence of carotid body tumor is rare; few disperse cases have been reported during the last couple of centuries followed by the Marchand report in 1891 (7). Mayo in a clinical report, enormously attempted to collect data from 153 cases during 50 years (8). A clinical presentation is often nonspecific and may only consist of a slowly-growing mass in the higher jugular-carotid region. In our study, we also found a nontender lateral neck mass as a unanimous finding among the patients, emphasizing the suspicion in such soft tissue masses.
Although the exact gender distribution has not been determined yet, female predilection has been elucidated in high-volume studies. Recent studies have reported that CBTs are more common in females than males (5, 6). In our study, none of the 48 patients was found to have a history of familial or glandular neoplasia, but the number of females was 1.6 times more than males.
Chronic hypoxia has long been recognized as an etiology of CBT and other paragangliomas. About 35% of the cases have been attributed to living in high altitudes. Yet, the percentage of highlanders in our study (58.8%) was higher, compared to other reports (25%) (5, 6). Recent biogenetic discoveries have revealed that mutations in oxygen-sensing genes are another etiology accounting for approximately 35% of cases, and the effect of this etiology is probably additive (3-7, 9). Although CBTs usually exhibit as single lesions, multicentricity, usually accompanied with bilaterality, has been reported in approximately 5% of sporadic tumors in comparison with 25 - 33% of familial cases. Metastasis might be reported in almost 5% of patients; it should be considered that distant metastasis is highly uncommon. In this study, 5.8% of patients had bilateral CBT.
The treatment of choice for cervical paragangliomas remains to be a surgical approach, as it results in a high cure rate of 89 - 100% (8, 10). Preoperative cranial nerve deficits have been reported in up to 20% of patients with CBTs (11, 12). We also achieved a 94.11% cure rate, but with a smaller rate of preoperative cranial nerve palsy at 11.7%. Despite significant improvements in preoperative evaluation, surgical methods, and various intraoperative monitoring techniques that lead to almost complete elimination of mortality rate, morbidity with neurologic complications is still high (10 - 40%) (3-9). A preoperative diagnosis is mandatory, based on Doppler color flow imaging and angiography.
Injury to the hypoglossal nerve is a recognized complication after soft tissue surgery, e.g. branchial cyst or CBT excision, in the upper part of the anterior aspect of the neck (11). In our study only 3 (6%) patients experienced residual nerve palsy due to massive local invasion to the affected nerve and subsequent excision (the rate of major vascular complications has dropped from 30% in the 1960s to less than 1% in the most recent reports (12)). We only had one major vascular complication in our patients.
In our research and other literature, surgical management was the treatment of choive for these tumors; it revealed no postoperative cerebrovascular accident and limited complications secondary to unavoidable nerves sacrifice. Radiation therapy was only performed in particular cases: surgery contra-indications and nonresectable cases. Available reports (13, 14), Davidge-Pitts and Pantanowitz, established a positive correlation between tumor size and tumor class, based on the Shamblin classification. They demonstrated that tumors of 4 cm or more (class 2 or 3 Shamblin tumors) might result in carotid arteries encasement either partially or completely.
Recently, some reports have been published about the use of radiotherapy as the treatment of choice for paragangliomas (15-17). Hinerman and colleagues quoted a 96 - 100% tumor control rate after radiation therapy of cervical paragangliomas using 45 Gy (18). However, surgery is still the first choice for treatment of these tumors, and also radiation therapy prior to surgery makes operative treatment more difficult. Due to replacement of the tumor with fibrosis connective tissue, evaluation of complete radiotherapic response such as macroscopic growth arrest of the CBT, might be challenging (10-16). Furthermore, vascular structure of the tumor is the predominant component responsible for radiotherapic response and might be replaced with fibrosis connective tissue during the therapeutic course. A potentially malignant tumor has been reported in 3 - 5% of cases (14-21).
The treatment of choice, surgical approach versus radiotherapy for cervical paragangliomas, remains a challenging debate as several variables including age and tumor size and site should be considered.
In general, surgery is a better treatment for patients. It is still the mainstay of therapy, but conventional or stereotactic radiotherapies seems to be very safe options for very large or inoperable tumors. Routine application of preoperative tumor embolization (22), as well as the potential advantages of tumor vasculature shrinkage and limitation of blood loss have not been established yet, which might superimpose the neurologic complications accompanied with the accidental reflux of the particulate matter into the ophthalmic or cerebral circulation (13, 23-25). Early resection of the carotid body tumors, when they are small in size, can facilitate their resection. Preoperative embolization of large tumors can facilitate resection and minimize blood loss during the operation. If operative resection cannot be performed because of internal carotid artery involvement, radiation can be used as a second alternative. We advocate resection of all tumors once they are discovered in appropriate patients.