1. Context
Non-alcoholic Fatty Liver Disease (NAFLD) includes a spectrum of liver cell damage. The accumulation of fat in hepatocyte (simple fatty liver or bland steatosis) is the first step in the course of disease. Inflammatory reactions (steatohepatitis) occur with the progression of disease (1). This process can eventually lead to end stage liver disease and hepatocellular carcinoma (2). NAFLD is a common cause of chronic hepatitis (1). The prevalence of Non-alcoholic Steatohepatitis (NASH) in a sample of general population of Iran is reported to be around 2% (3). The prevalence of viral hepatitis is decreasing in Iran; meanwhile the NAFLD prevalence seems to be increased due to the epidemic of obesity (4-6). NAFLD should be suspected in patients with any form of chronic liver disease including autoimmune hepatitis (7). The early diagnosis and proper management of NAFLD is necessary to delay disease progression. The most of referred patients for the evaluation of NAFLD are diagnosed initially either by an imaging study (liver ultrasonography) or by an increase in serum aminotransferase levels.
2. Evidence Acquisition
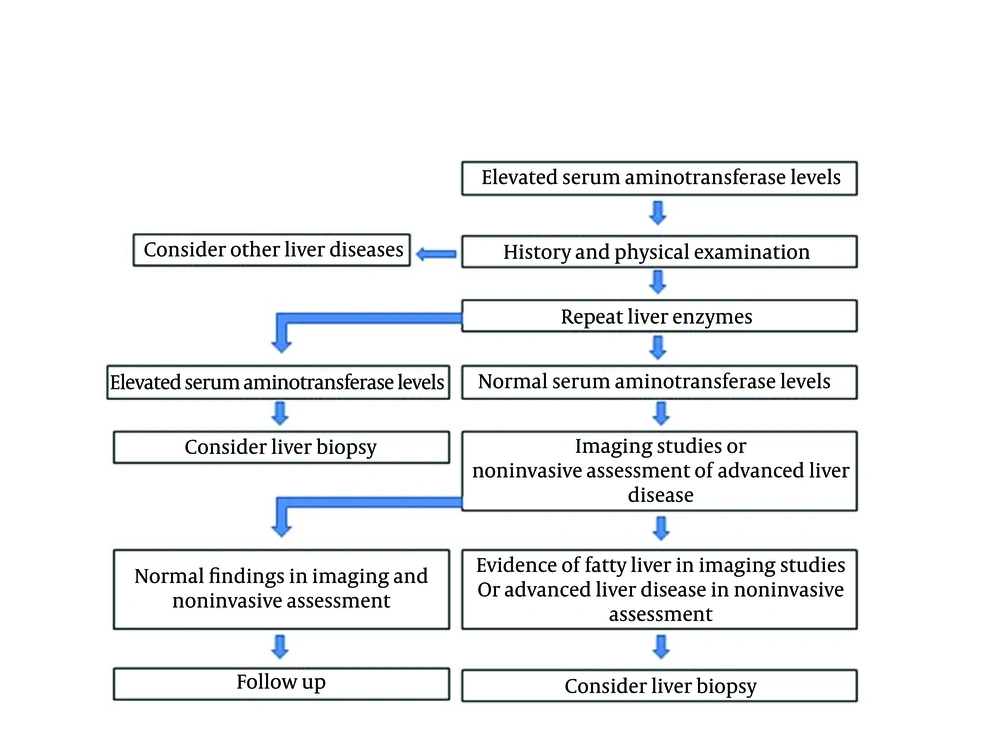
The documentation of diagnosis is the first step in the management of these patients. NAFLD is yet a diagnosis of exclusion. Diagnosis is based on the patient history, physical examination, laboratory findings, and imaging studies. After excluding causes of liver cell damage, the patients with persistent aminotransferase levels and well-defined criteria for fatty liver at ultrasonography are presumed to have NAFLD. Documentation of NAFLD by liver biopsy is not obligatory in routine practice. However, when concomitant liver disease exists, the diagnosis of NAFLD is questionable without liver biopsy. Since NAFLD is considered as the hepatic manifestation of metabolic syndrome, its identification is easily confirmed by the presence of insulin resistance components. Liver biopsy is already considered as the gold standard method to diagnose and evaluate the extent of liver parenchymal damage in NAFLD. The invasiveness and possible complications of this modality, have limited its use. Considering the limitations of diagnostic methods, selection of the best diagnostic approach has become a conflicting issue. A practical approach is shown in Figure 1 for diagnosing NAFLD.
3. Results
3.1. Patient History
The physician should notice to the special clues in the patient history. This helps the physician to differentiate NAFLD from the other causes of chronic liver diseases that have similar clinical manifestations. Most of the NAFLD patients are asymptomatic, but some might complain of malaise, fatigue, and right upper quadrant discomfort (8). Strong association between NAFLD and diabetes mellitus, obesity, and hyperlipidemia were reported (9 - 11). NAFLD is already considered as the hepatic manifestation of metabolic syndrome (12). Presence of diabetes mellitus or its vascular complications (Ischemic heart disease, cerebrovascular accident, retinopathy, neuropathy, nephropathy, and diabetic foot), obesity, hypertension, hyperlipidemia, and hyperuricemia, indicate the presence of metabolic syndrome (13 , 14). A list of conditions associated with NAFLD is shown in Table 1.
| Cardiovascular disease |
| Polycystic ovary syndrome |
| Obstructive sleep apnea |
| Total parenteral nutrition with glucose |
| Starvation |
| Rapid weight loss |
| Hypothyroidism |
| Small bowel resection |
| Gastroplasty for morbid obesity |
| Biliopancreatic diversion |
| Jejunal bypass |
| Partial lipodystrophy |
| Abetalipoproteinemia |
| Jejunal diverticulosis |
| Bacterial overgrowth syndrome |
Conditions Associated With Non-Alcoholic Fatty Liver Disease
Low socioeconomic status, poor hygiene, and living in endemic areas for Hepatitis A Virus (HAV) and Hepatitis E Virus (HEV) infections predispose the patients to these viral infections (5, 15, 16). History of tattooing, injection drug use, hemodialysis, blood transfusion, surgical procedures, maternal Hepatitis B Virus (HBV) or Hepatitis C Virus (HCV) infection, working in health care centers, and unsafe sex are the risk factors for HCV and HBV infections(17-24). The neuropsyachiatric symptoms (speech and handwriting change, abnormal movements, tremor, declining school performance, personality and behavioral changes, impulsiveness, labile mood, paranoia, schizophrenia, and depression) guide to the diagnosis of Wilsons disease (25). The onset of diabetes mellitus with hyperpigmentation in patients that need multiple blood transfusions (thalassemia major) points to the diagnosis of hemochromatosis (26). Arthralgia, oral ulcers, and skin rash may guide the physician to autoimmune hepatitis (27). Generalized pruritus, jaundice, dark urine and pale stools, the symptoms of fat soluble vitamin deficiency (bone pain, night blindness, easy bruising) might be seen in chronic cholestatic liver disease. Severe systemic co-morbidities or neoplasm may influence liver function tests. In one study, the most prevalent causes of elevated liver enzymes in hospitalized patients were systemic infections and drug induced liver injury (28). Stauffer's syndrome is a rare paraneoplastic manifestation of renal cell carcinoma that is characterized by elevated alkaline phosphatase, erythrocyte sedimentation rate, α-2-globulin, and γ-glutamyltransferase, thrombocytosis, prolongation of prothrombin time, and hepatosplenomegaly, in the absence of hepatic metastasis and jaundice (29).
3.2. Medication History
Medications can affect liver function tests. Sometimes there is transient elevation in aminotransferase levels after the initiation of medication (adaptive response) (30). Various medications have hepatotoxicity and might show hepatocellular or cholestatic type liver damage. Fatty change may also occur due to the medication use (Table 2) (31). For the proper diagnosis of NAFLD, an accurate medication history is necessary to exclude drug induced liver injury. Those with the history of any hepatotoxic medication use during the past three months should be considered as having drug-induced liver injury.
| Glucocorticoids |
| Synthetic estrogens |
| Tetracycline |
| Minocycline |
| Amiodarone |
| Tamoxifen |
| Antiretroviral agents |
| Perhexiline maleate |
Drugs That Might Cause Fatty Liver Disease
3.3. Habitual History
The histological and biochemical findings in alcoholic hepatitis are very similar to NAFLD. Only precise history of alcohol consumption can differentiate alcoholic hepatitis from NAFLD. Alcohol consumption more than 20 gram per day in men or 10 gram per day in women is in favor of alcoholic hepatitis (32). The coincidence of alcoholic hepatitis with NAFLD may exist. This occurs when an alcoholic patient has concomitant metabolic syndrome.
3.4. Family History
The familial clustering of HBV and HCV infections are reported in the literature (33). Paternal HBV or HCV infection and deaths related to these infections should be investigated in the family history. The relativeness of parents may result in some rare autosomal recessive diseases (Wilson’s disease and alpha one antitrypsin deficiency). Evidences of metabolic syndrome components (history of diabetes, hypertension, hyperlipidemia and obesity) and deaths related to metabolic syndrome should be evaluated in the family history.
3.5. Physical Examination
Physical examination is not a sensitive tool for the early detection of NAFLD or the evaluation of hepatic function. Most of the patients have unremarkable findings in physical examination except for hepatomegaly. Signs of cirrhosis (palmar erythema, white nail, spider angiomata, gynecomastia, muscle wasting and hepatic encephalopathy) or portal hypertension (splenomegaly, ascites, variceal bleeding) might be seen in the advanced stages of disease (34). Palmar fasciitis (Dupuytren's contracture), Wernicke encephalopathy, dementia (Korsakoff's syndrome), and parotid enlargement point to the diagnosis of alcoholic hepatitis (35). Arthritis, oral ulcer, hypothyroidism, and other autoimmune associated diseases guide the physician to autoimmune hepatitis (27).Parkinsonian syndrome, tremor, ataxia, dystonic syndrome, and KF ring, are in favor of Wilsons disease (25). Xanthoma, xanthelasma, osteomalacia, osteopenia, and echymosis might be present in primary biliary cirrhosis (PBC) (36).
Components of metabolic syndrome should be investigated for evaluating the severity of NAFLD. Hypertension and obesity are among the important components that must be evaluated on physical examination. Body Mass Index (BMI) and Waist to Hip Ratio (WHR) are helpful in the evaluation of insulin resistance syndrome (37-39). WHR and abdominal fat content are related to the complications and the survival of patients with metabolic syndrome (40). Special attention should be paid for detecting the micro and macrovascular complications of diabetes mellitus (diabetic retinopathy, neuropathy, nephropathy, ischemic heart disease, cerebrovascular accident, and diabetic foot) in the physical examination.
3.6. Laboratory Investigations
Laboratory investigations are used for the diagnosis of NAFLD by ruling out the other causes of liver damage. A panel of laboratory parameters is already available for the exclusion of other known causes of liver damage. (Table 3) Laboratory data is also applied for the evaluation of hepatic function. Alanine aminotransferase (ALT) and Aspartate aminotransferase (AST) are among the most common serum parameters that indicate liver cell injury. Their values might be normal in simple fatty liver disease (41). Simple fatty liver disease is considered as the early stage of NAFLD with accumulation of fat in hepatocytes and minimal inflammatory reaction in liver parenchyma. This stage can only be detected with imaging studies showing fatty infiltration in the liver. NASH seems to be the more advanced stage of disease than the simple fatty liver. Inflammatory reactions with concomitant liver cell injury are present in NASH. Therefore, serum ALT and AST values are increased in NASH (41). Serum aminotransferase values show fluctuations during the course of disease. Their values might be near normal in early stage and sometimes in end stage liver disease (42). The healthy ranges of serum aminotransferase are proposed to be lower than the cut-off points recommended by the manufacturers' kit (43). Considering the healthy range of ALT that is lower than the currently used reference range by the laboratories (40 units per liter) and the fact that gender influences ALT levels, the application of ALT healthy ranges will result in discovering more patients with mild fatty liver disease (44). AST is an intracellular enzyme that is released into serum when cell death occurrs. It is not a specific marker for liver cell death and may be elevated in other tissue injuries (45). Therefore, it seems that ALT and AST concentrations alone are not sensitive enough for the diagnosis or evaluation of liver function in NAFLD especially in the early stage of disease.
| Hepatitis B surface antigen, Hepatitis B surface antibody, Hepatitis B core antibody |
| Hepatitis C virus antibody |
| Ferritin, serum iron, Total iron binding capacity |
| Ceruloplasmin, 24 hour urine copper |
| Gamma globulin level, anti-mitochondrial antibody, antinuclear antibody |
| Anti-tissue transglutaminase antibody (Ig A, Ig G) |
| Alpha one antitrypsin |
Laboratory Investigations for the Evaluation of Abnormal Liver Function Tests
The AST/ALT ratio (AAR) is usually less than one in NAFLD. This is lower than the ratio observed in alcoholic hepatitis. In alcoholic hepatitis, the ratio is frequently above two (46). Serum alkaline phosphatase, γ-glutamyltransferase, 5' nucleotidase, bilirubin, prothrombin time, and albumin concentrations remain normal until very late in the course of disease (46). The changes in routine laboratory concentrations are not specific for NAFLD and might be detected in any form of liver damage. Laboratory investigations are not sensitive enough for the evaluation of NAFLD severity and prognosis. However, there are reports in literature that increased ALT and triglyceride (TG) and decreased High Density Lipoprotein (HDL) levels are associated with the severity of NAFLD (47-50). The TG levels greater than 150 mg/dl and HDL level less than 45 mg/dl for men and 50 mg/dl for women are considered abnormal (51). It seems reasonable to check for the components of insulin resistance syndrome [such as fasting plasma glucose, blood sugar two hours post prandial, hemoglobin A1C, serum insulin, TG, Cholesterol, HDL, low density lipoprotein, and uric acid] to define the severity of NAFLD (37). Increased ferritin level is reported in NAFLD patients (52). The HFE mutation analysis is recommended to rule out hemochromatosis when increased serum ferritin level exists. Low titers of auto-antibodies associated with autoimmune hepatitis are reported in advanced stage of NAFLD (53). The role of these antibodies on the pathogenesis of NAFLD is not clear.
3.7. Imaging Studies
Imaging studies are safe and acceptable modalities for the diagnosis of NAFLD (54). They have become popular for estimation of the severity of NAFLD and the diagnosis of patients in early (pre-clinical) stage of disease, even before the liver function tests show any abnormality. However, they are not sensitive for differentiating inflammation from fibrosis in the liver (54).
3.7.1. The Role of Ultrasonography
Many physicians consider the ultrasonography (US) of liver as a screening tool for the diagnosis of NAFLD. A fatty liver scatters the beam of ultrasound more than a normal liver; therefore, the fatty liver appears hyperechogenic (55). Because there is no absolute echogenicity that denotes liver fat, the comparison of echogenicity is required with internal organs known to be void of fat, such as the kidneys or spleen (56). Although this imaging modality is a safe and acceptable method for the diagnosis of NAFLD, it has some limitations. This method is operator dependent and inter-observer variability exist in the reports of liver US. B mode US cannot detect small changes in liver fat content over time, so it cannot be applied for the follow up of NAFLD patients (57). The method cannot distinguish fibrosis from fatty change (58). Sometimes the fat accumulation in liver is not distributed homogenously and the localized fatty change may be masquerade as hepatic mass lesion (59). The sensitivity and specificity of US in detection of NAFLD is decreased in obese patients (57). It seems that liver US alone is not suitable for the diagnosis of NAFLD. However, the combination of US and serum parameters might increase the diagnostic accuracy for the early diagnosis of NAFLD in complementary to laboratory investigations (60).
This method can also evaluate the severity of liver involvement by using visual assessment of hepatic echogenicity (55). To define the severity of NAFLD, the US findings are graded from one to three according to the echogenicity of the liver. In grade one (mild), echogenicity is slightly increased, with the normal visualization of diaphragm and intra-hepatic vessel borders. In grade two (moderate), echogenicity is moderately increased, with the slightly impaired visualization of diaphragm or intra-hepatic vessels. In grade three (severe), echogenicity is markedly increased, with the poor or no visualization of diaphragm, intra-hepatic vessels, and the posterior portion of right lobe (54). The US grading of NAFLD is based on visual analogue scale. This system has limitations in differentiating moderate from severe groups and there is overlap between the US grading (47). Sometimes the patients with the borderline US findings of moderate group or severe group might be misclassified as to either group. To overcome this shortcoming, the assessment of hepatic vein Doppler waveform and hepatic artery resistance index by using color Doppler US are newer techniques that recently have come to interest for the evaluation of NAFLD severity (61).
3.7.2. The Role of CT Scan and MRI
The data obtained from the patients who underwent liver resection for malignancy showed the followings: 1) Non-contrast enhanced CT scan cannot exclude significant steatosis particularly in obese patients. 2) A contrast enhanced CT scan does not accurately define the steatosis. 3) A normal MRI excludes significant steatosis, but abnormal findings are not indicator of fatty liver (62).
3.7.3. The Role of Proton Magnetic Resonance Spectroscopy
Proton magnetic resonance spectroscopy is a new imaging modality that can predict the hepatic fat content quantitatively. It is already considered as the gold standard non-invasive method for the detection of NAFLD. Kotronen et al developed a liver fat score using proton magnetic resonance spectroscopy that predicted increased liver fat content with the sensitivity of 86% and specificity of 71% (63).
3.8. The Panel of Biomarkers and Scoring Systems for Diagnosis and the Estimation of Severity in NAFLD
Dunn et al. showed that the mean corpuscular volume, AAR, BMI, and gender were the most important variables that separated patients with Alcoholic Liver Disease (ALD) from NAFLD. These variables were used to generate the ALD/NAFLD Index (ANI), with ANI of greater than zero incrementally favoring ALD and ANI of less than zero incrementally favoring a diagnosis of NAFLD (64).
Several biomarkers have been studied for the evaluation of inflammation (such as CRP, IL6, TNF a, plasma pentraxin 3), oxidative stress (superoxide dismutase, glutathione peroxidase activity and vitamin E level), and fibrosis (such as Transforming growth factor B, type 4 collagen 7S domain, hyaluronic acid, polypeptide specific antigen, tissue inhibitors of metalloproteinases, endothelin 1, cytokeratin 18) in NAFLD (65- 71). Most of these biomarkers are not specific for NAFLD. Moreover, they are not yet validated in a large number of patients for this purpose. The result of a new study showed that adiponectin, leptin, and ghrelin were associated with more severe NAFLD. A formula combining the three cytokines showed good accuracy for NASH (72). Multiple scoring systems are described to define the severity of liver steatosis, inflammation, and fibrosis in NAFLD (70, 73 - 78) (Table 4). These scoring systems should be validated in a large number of NAFLD patients before they can be applied in common practice. Substitution of these alternatives for liver biopsy seems promising in future.
| Identification of steatosis | |
| NAFLD liver fat score | Presence of diabetes mellitus |
| Fasting serum insulin | |
| AST | |
| AST/ALT ratio | |
| Fatty liver index | Body mass index |
| Waist circumference | |
| Triglyceride | |
| γ-glutamyl transferase | |
| Visceral adiposity index | Body mass index |
| Waist circumference | |
| Triglyceride | |
| High density lipoprotein | |
| Identification of inflammation | |
| NASH test | Total Bilirubin |
| γ-glutamyl transferase | |
| α2 macroglobulin | |
| Apolipoprotein A1 | |
| Haptoglobulin | |
| ALT | |
| HAIR test | Hypertension |
| ALT | |
| Insulin resistance | |
| Palekar model | Age |
| Gender | |
| AST | |
| Body mass index | |
| AST/ALT ratio | |
| Hyaluronic acid | |
| Identification of fibrosis | |
| NAFLD fibrosis score | Age |
| Hyperglycemia | |
| Platelet count | |
| AST/ALT ratio | |
| Body mass index | |
| Albumin | |
| FIB 4 index | Age |
| AST | |
| ALT | |
| Platelet count | |
| Fibrotest (BioPredictive) | γ-glutamyl transferase |
| Haptoglobin | |
| Bilirubin | |
| ALT | |
| Apolipoprotein A | |
| α2 macroglobulin | |
| Fibro Spect | Hyaluronic acid |
| Tissue inhibited matrix metalloproteinase inhibitor 1 | |
| α2 macroglobulin | |
The Panel of Markers and Scoring Systems for the Evaluation of NAFLD Severity
3.9. The Role of Liver Biopsy
Liver biopsy is already considered the gold standard method for the diagnosis of NAFLD. It is used when definitive clinical and laboratory findings are absent for ruling out the other causes of chronic hepatitis. Liver biopsy is not necessary for the diagnosis of NAFLD when the clinical and paraclinical findings are apparently in favor of NAFLD diagnosis and other causes are excluded. The typical histological findings in NAFLD are shown in Table 5. Clinical findings, imaging studies and laboratory investigations have limitations for predicting the severity of disease. Liver biopsy is already considered as the method of choice for evaluation the extent of steatosis, inflammation, and fibrosis in NAFLD. However, the possible risks and invasiveness have limited its use in common practice. Several histology scoring systems are introduced for defining the disease severity and response to treatment in chronic hepatitis. NAFLD Activity Score (NAS) seems to be more specific than the others in NAFLD. This scoring system evaluates macrovesicularsteatosis, lobular inflammation, hepatocyte ballooning, and perisinusoidal fibrosis (Table 6). Score five or greater is consistent with NASH, and score two or less is consistent with simple fatty liver. It is not advised to repeat liver biopsy for patients with simple fatty liver.
| Liver steatosis |
| Hepatocyte ballooning degeneration |
| Mixed acute and chronic lobular inflammation |
| Perivenular and perisinusoidal fibrosis |
| Zone 3 accentuation |
| Mallory hyaline bodies |
| Vacuolated nuclei in periportal hepatocytes |
| lobular lipogranuloma |
| PAS-diastase-resistant Kupffer cell |
| Pericellular fibrosis in advanced stages |
| Chronic portal inflammation and fibrosis |
Characteristic Histological Findings in non-Alcoholic Fatty Liver Disease
| Steatosis | |
| Grade | Low to medium-power evaluation of parenchymal involvement by steatosis |
| < 5% | |
| 5% - 33% | |
| > 33% - 66% | |
| > 66% | |
| Location | Predominant distribution pattern |
| Zone 3 | |
| Zone 1 | |
| Azonal | |
| Panacinar | |
| Microvesicular steatosis | Contiguous patches |
| Not present | |
| Present | |
| Fibrosis | |
| Stage | None |
| Perisinusoidal or periportal | |
| Mild, zone 3, perisinusoidal | |
| Moderate, zone 3, perisinusoidal | |
| Portal / periportal | |
| Perisinusoidal and Portal / periportal | |
| Bridging fibrosis | |
| Cirrhosis | |
| Inflammation | |
| Lobular inflammation | Overall assessment of all inflammatory foci |
| No foci | |
| < 2 foci per 200 x field | |
| 2-4 foci per 200 x field | |
| > 4 foci per 200 x field | |
| Microgranulomas | Small aggregates of macrophages |
| Absent | |
| Present | |
| Large lipogranulomas | Usually in portal areas or adjacent to central veins |
| Absent | |
| Present | |
| Portal inflammation | Assessed from low magnification |
| None to minimal | |
| Greater than minimal | |
| Liver Cell Injury | |
| Ballooning | None |
| Few balloon cells | |
| Many cells / prominent ballooning | |
Non Alcoholic Fatty Liver Disease Histology Activity Score (NAS)
3.10. Transient Elastography
Measurement of liver stiffness by transient elastography is a promising non-invasive method for excluding advanced fibrosis (70). This method evaluates liver stiffness using pulse-echo ultrasound. A larger part of liver is evaluated in this method than liver biopsy. The main limitation of its use is the interference by steatosis with wave velocity (70). This method might be unreliable in obese patients due to the technical reasons. Since a significant number of NAFLD patients are obese, its usage might be limited by the current equipments. Further investigations are needed before routine application of this method for the diagnosis and follow up of NAFLD patients.
4. Conclusions
A practical approach for the diagnosis of a patient suspected to have NAFLD was proposed in this review. NAFLD is an increasing cause of liver damage. It is considered as the hepatic manifestation of metabolic syndrome. This disease contains a spectrum from simple fatty infiltration to steatohepatitis. The latter might lead to end stage liver disease. Diagnosis is based on excluding other causes of chronic hepatitis concomitant with evidence of fatty liver in imaging studies. Ultrasonography is commonly used as a screening tool for this purpose; however, obesity limits its accuracy in detecting NAFLD patients. The role of non-invasive methods for diagnosis and estimation of disease severity remains controversial. Liver biopsy is already the gold standard method for this purpose.