1. Background
The aromatic organic compound, mitoxantrone (an anthraquinone, anthracenedione or dioxoanthracene) is useful in the secondary progressive phase (SP) of multiple sclerosis (MS) (1). Triggered astrocytes generate nitric oxide (NO) and other molecules, such as interleukin 12 (IL12), interleukin 23 (IL23), T helper 1 (Th1) and T helper 17 (Th17), which can be noxious to oligodendrocytes, therefore potentially contributing to the pathology associated with MS. Monocyte chemoattractant protein-1 (MCP-1) is a chemokine supposed to adapt the passage of monocytes to provocative injuries, found in the central nervous system of MS patients. Mitoxantrone inhibits lipopolysaccharide induction of NO, tumor necrosis factor α (TNF-α), interleukin 1β (IL1β), and MCP-1 by key astrocytes. Mitoxantrone also inhibited IL12 and IL23 synthesis by these cells (2).
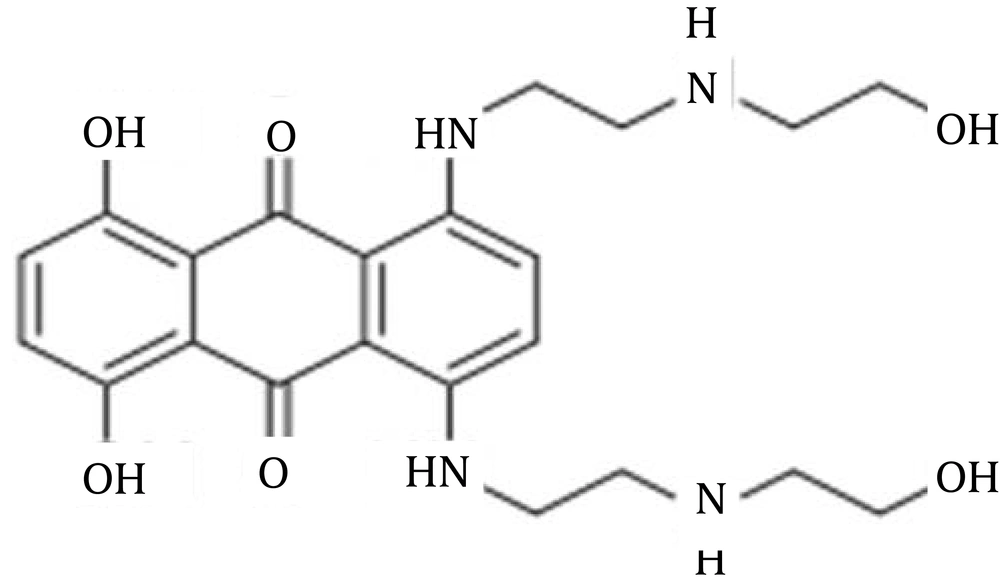
The assumption of initiation treatment pursued by extended-period continuation management, in MS, has concerned significant awareness. While the extent of management alternatives related to different phases of MS, such as relapsing-remitting (RR), is increasing continuously, substitutes are uncommon in patients with SP (3). The administration of mitoxantrone, as the stimulation remedy behind an immunomodulatory drug, is of attentive interest. In addition to mitoxantrone, in North America and, interferon beta-1b (IFNβ-1b) and beta-1a (IFNβ-1a) are accepted for prescription in Europe. Glucocorticosteroids, azathioprine, intravenous immunoglobulins and cyclophosphamide, even though not established in guidelines, are frequently employed in SPMS (1-4). Previous publications reported 6-month course of mitoxantrone, followed by continuation treatment, such as immunomodulatory drugs, results in a speedy decrease in disease action and a constant syndrome management for at least 5 years. In addition, induction with mitoxantrone, followed by maintenance treatment, affords better disease control than monotherapy with an IFNβ (5). Scott et al. in 2004, reported that mitoxantrone 12 mg/m2 administered once every 3 months, for 2 years, could provide significant improvements in neurological disability ratings, including Kurtzke expanded disability status scale (EDSS), ambulatory index (AI) and standardized neurological status (SNS) scores (6). Chan et al. in 2013, reviewed therapy-related acute leukemia in patients under therapy with mitoxantrone and highlighted the need for close hematologic monitoring during and after therapy (7). In another publication, reported by Hofmann et al. in 2013, it was confirmed that mitoxantrone treatment in MS is connected with long-term major potential harms, like leukemia and cardiotoxicity (8). Figure 1 shows the chemical structure of mitoxantrone.
As shown in Table 1, the bioavailability (F) of novantrone or mitoxantrone is not available. It is metabolized by Cytochrome P450 2E1 via the liver. Its half-life is 75 hours. The drug is bound to plasma proteins by 78%. It is mainly eliminated via the kidneys (1).
| Mitoxantrone Kinetic Parameters | |
|---|---|
| F | N/A |
| PB | 78% |
| M | H (CYP2E1) |
| T1/2 | Variable |
| E | R |
a Abbreviations: CYP, Cytochrome P; E, Excretion; F, Bioavailability; H, Hepatic; N/A, Not available; M, Metabolism; PB, Protein Binding; R, Renal; T1/2, Half-Life.
The main adverse effects are nausea, vomiting, hair loss, heart damage, and immunosuppression (8-14). The study by Filippini et al. described a decrease in relapses, when compared with placebo, by using mitoxantrone (14). The low risk of cardiotoxicity, within one year of treatment, and an efficient dose of 12 mg/m2 in an Iranian MS population has been reported by Hamzehloo and Etemadifar (15, 16). The drug has a rapid initial (alpha with t1/2 of 6 - 12 minutes), intermediate (beta with t1/2 of 1.1 - 3.1 hours) distribution phase and a much lower elimination (gamma) phase. Mitoxantrone has a high affinity for tissue, with a distribution volume of up to 2248 L/m2. Mitoxantrone persists in tissues for prolonged periods and was reported to be measureable in autopsy tissue from patients who last received the drug up to 9 months before death. At concentrations of 10 - 10,000 ng/mL, the drug was 70% - 80% bound to plasma proteins, in dogs. Elimination of mitoxantrone occurs predominantly through biliary excretion and may be impaired in patients with hepatic dysfunction or third space abnormalities (e.g. ascites). The mean E1/2 of mitoxantrone ranged from 23 - 215 hours. Renal clearance accounts for 10% of the total clearance of the drug. Total clearance of mitoxantrone ranged from 13 - 34.2 L/h/m2 and renal clearance from 0.9 - 2.7 L/h/m2 (1, 17, 18).
2. Objectives
The aim of this preliminary study was to investigate evidence-based effects of mitoxantrone prescription in patients with MS.
3. Patients and Methods
This cross-sectional study of 71 patients was conducted by the deputy of research in the Isfahan university of medical sciences, and included patients attending MS clinics during from 2010 to 2012. Demographic variables, including sex and age, in addition to pharmacological variables related to receiving or not receiving mitoxantrone, receiving or not receiving pulse therapy using methylprednisolone (MP), were recorded in dBase (dBase LLC, Binghamton, NY, USA) and analyzed using SPSS for Windows (SPSS Inc., Chicago, IL, USA).
4. Results
Table 2 shows demographic, clinical and pharmacological data related to each individual patient with MS.
| Patients’ Code No | Frequency of Mitoxantrone Doses | Incidence of MP Pulsetherapy | According to Published Report The Risk of Side-Effects |
|---|---|---|---|
| 4 (M; 40 y), 17 (F; 39y), 27 (F; 51y), 28 (M; 40 y), 29 (F; 56y), 32 (M; 61 y), 38 (F; 37y), 51 (F; 41y), 52 (M; 54 y), 54 (F; 24y). | All received one dose of Mitoxantrone | Codes 28, 29, 38 received four times, Code 52 received six times pulsetherapy and others just one time. | Depends on individual variation |
| 35 (F; 45y), 24 (M; 33y), 39 (M; 37y) | All received two dose of Mitoxantrone | Code 35 received five times, Code 24 and 39 received seven to nine times MP pulsetherapy respectively. | ↑ |
| 30 (F; 46y) | She received five dose of mitoxantrone | This woman visited MS clinic from 19/12/2009 - 26/01/2011 eight times for MP pulsetherapy. | ↑ |
a Abbreviations: F, Female; M, Male; MP, Methylprednisolone; y, year old; ↑, Increasing.
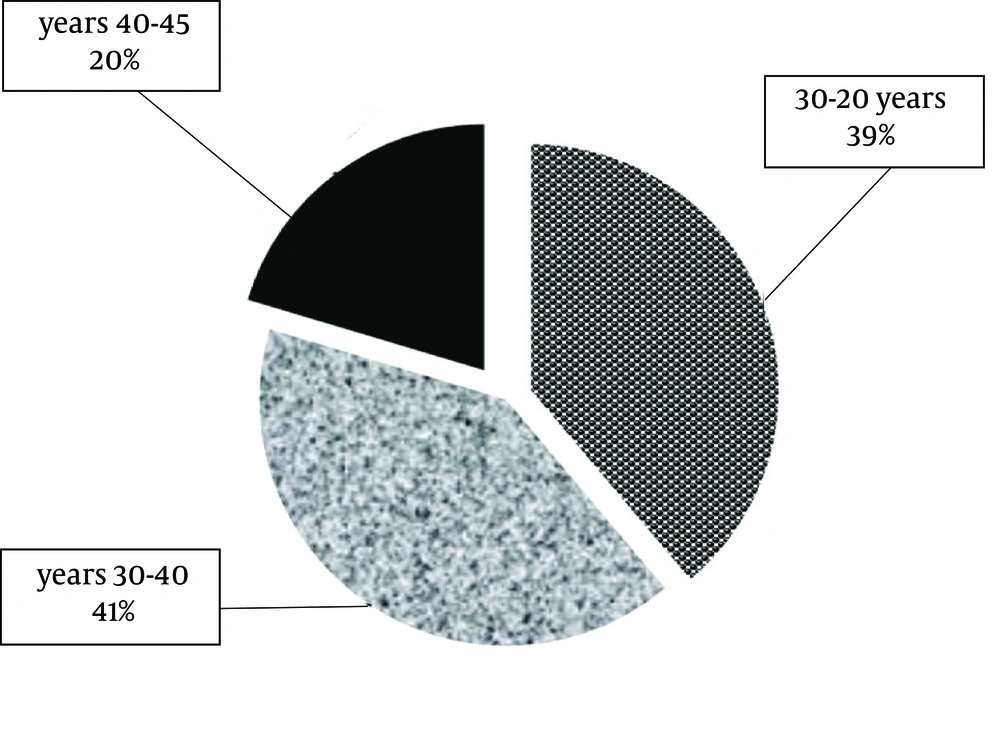
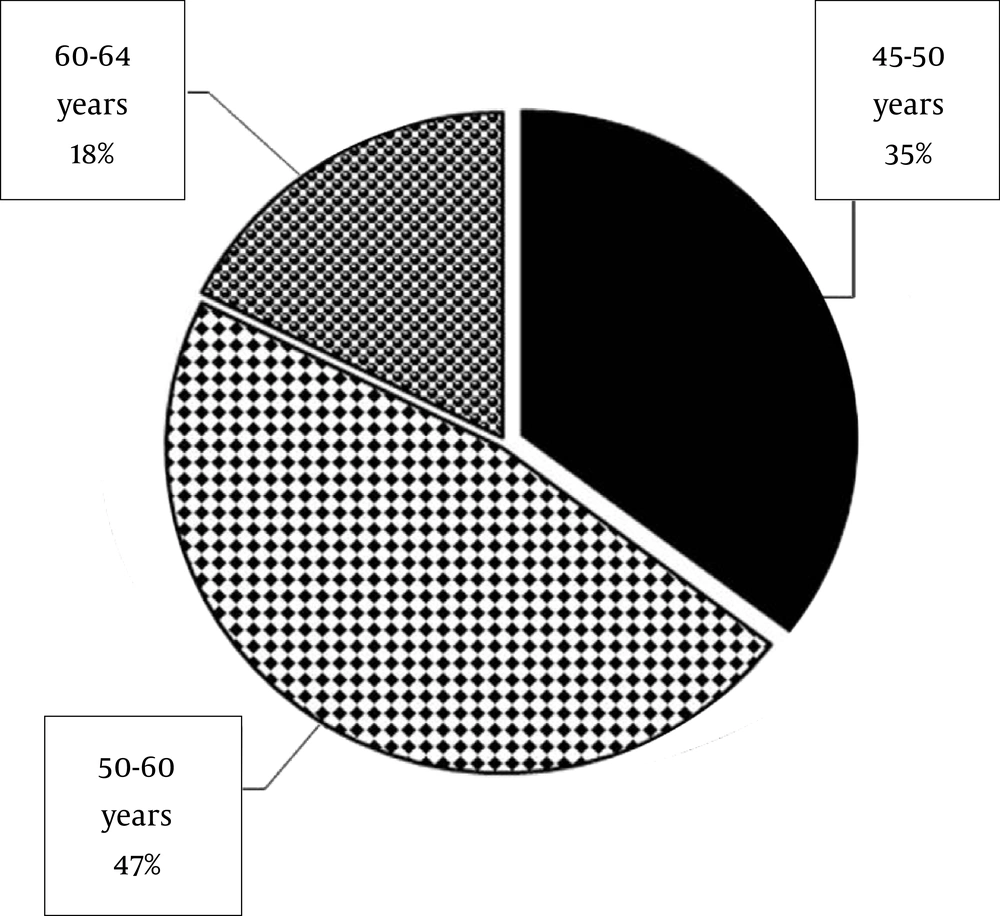
The females (n = 54; Figure 2) to males (n =17; Figure 3) distribution showed a sex ratio of 3.17: 1. With a range of 20 - 64 years old, the mean age of all patients (n = 71) was 37.1 years old. The mean age of females was 32.2 years old (ranged 20 - 45 years old) and the mean age of males was 52.7 years old (ranged: 45 - 64 years old). Females were younger than the males.
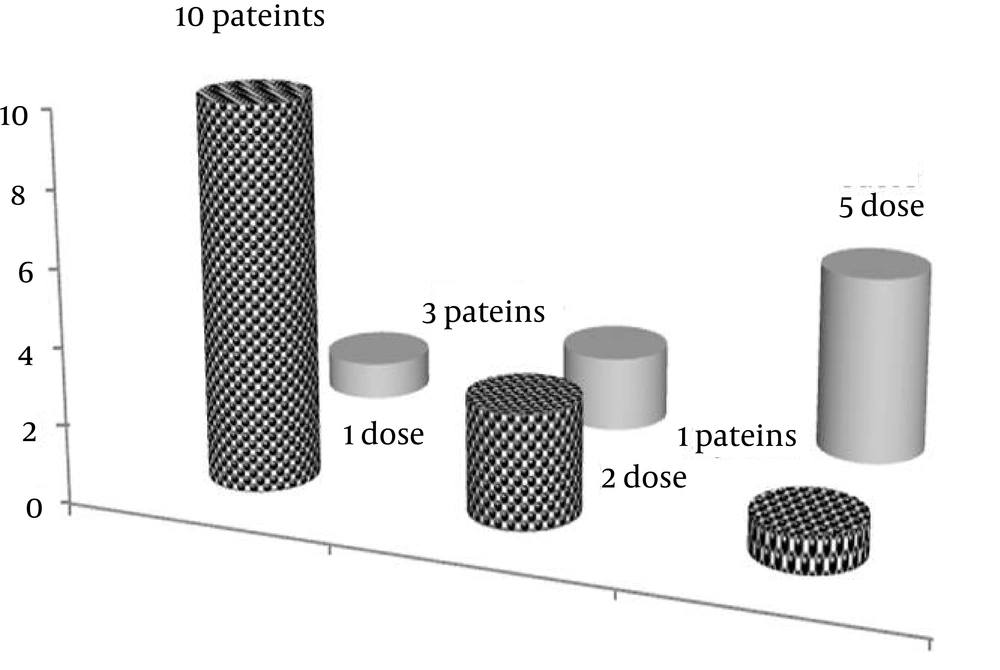
Of the total population studied (n = 71), 14 patients (comprised of eight females and six males) were those who received MP pulsetherapy on 52 occasions (19.7%).The mean age in these patients was 43 years, with a range of 24 - 61 years old. The mean number of visits per patients for MP pulsetherapy was four times, with a maximum of nine times. The mean frequency of mitoxantrone doses, received by each individual, was two with a range of one to five. Figure 4 shows that the frequency of mitoxantrone (2 mg/mL) prescribed for each patient could be ranked as follows: five times (n = 1), two times (n = 3), one time (n = 10). In eight out of 71 patients (approximately 13%), the number of MP pulsetherapy was ≥ four times.
Patient with code no. 30 is a 46-year-old housewife-lady, who visited the MS clinic eight times. As shown in Figure 4, she received MP pulsetherapy from 19/12/2009 to 26/01/2011, and the mitoxantrone was prescribed for her in five intervals at doses of 138 (second), 29 (third), 121 (fourth) and 95 (fifth) days, respectively.
5. Discussion
Mitoxantrone, or novantrone, is used as an anticancer agent and, also, in SPMS [5]. Previous publications reported that mitoxantrone could reduce the extent of disability. The decrease in number of relapses per year, the percentage of patients free from relapses at one or 2 years and the number of patients with active lesions on magnetic resonance imaging, at 6 months or one year have been confirmed by previous studies (1-23).
In agreement with a previous study (19), the mean age of patients was 37.1 years, with a range of 20‒64 years. Besides the fact that the incidence of MP pulsetherapy was 19.7%, also, in 13% of the patients, the demand for such therapy was four times greater. With concern to this direction, the frequency of mitoxantrone doses, received by each individual, ranged from one to five. Three patients received two and one patient received five doses, suggested to be more conscious for preventing adverse effects, according to previous published reports (5-14). Previous reports confirmed that the risk of increasing therapy-related acute leukemia and cardiotoxicity should have commanded to treatment restrictions for mitoxantrone (6). Other adverse effects noted were amenorrhea, nausea and vomiting, alopecia and urinary tract infections. Studies, with longer follow-up, have raised concerns about the risk of systolic dysfunction and therapy-related acute leukemias. Mitoxantrone should be restricted in treating patients with worsening RRMS and SPMS and with obvious inflammatory activity (1-23).
Myelosuppression is the main item related to toxicity that, in most patients, should be a concern for dose limitation. There are considerable reports regarding to drug T1/2, ranging from 2.9 - 214.8 hours (15). Because of rapid distribution to tissues, its plasma levels decline very rapidly and, therefore, concentration declines within a few hours. Previous publications reported that even at 35 days after the prescription of 14C-drug, the amount was high in many tissues, including liver, pancreas, spleen, and heart, with about 15% of the administered dose still retained in the body (15, 17, 18, 20-23). By using more sensitive methods for detection of low amounts of drug, to as low as 1 ng/ml, the longest T1/2 was reported as 214.8 hours (15, 21). The study performed by Rossato et al. confirmed genotoxic, hemato- and direct hepatotoxicity effects of mitoxantrone. The hepatic levels of reduced and oxidized glutathione were increased and ATP hepatic levels were decreased (12). A current meta-analysis points to a therapy-related acute leukemia risk of around 0.81%, which is higher than previous reports, and also marks that cardiotoxicity could lead to treatment restrictions (6).
Finally, in Iranian population with MS, prescription of mitoxantrone should be considered with close monitoring of drug side effects. To prevent long-term adverse effects, such as the increased incidence of myelosuppression, leukemia and cardiotoxicity, studies concerning the mitoxantrone correlation with rate and severity of relapses are recommended.