1. Background
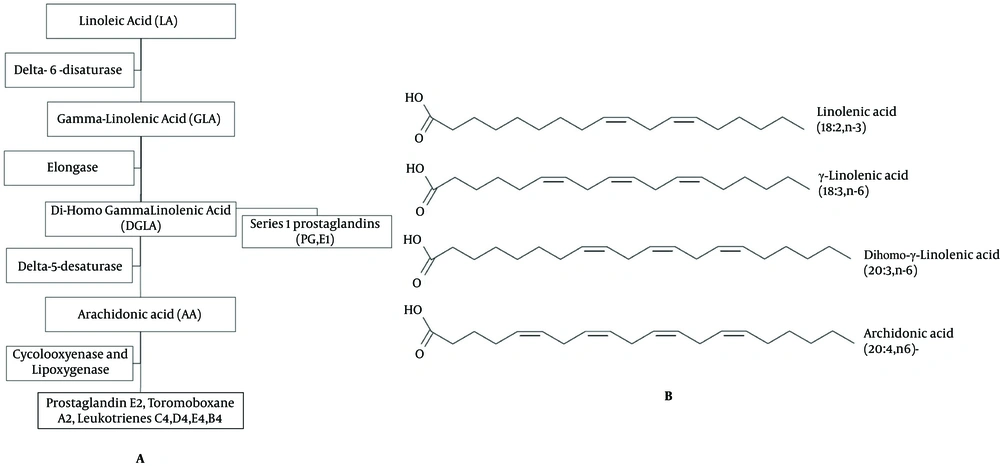
Nowadays, hysteroscopy can help the gynecologists to determine the uterine anatomy, physiology and pathology (1, 2). One of the main problems with operative hysteroscopy is passing the hysteroscope through internal cervical os when it is closed, particularly when using a resectoscope (1, 3, 4). The complications include laceration of cervix, creation of false tract, and perforation of uterus, viscera and vessels (1, 3-5). Priming agents for cervical ripening are effective in dilating the cervix. For example, Laminaria sticksis effective in dilating the cervix, but it is not suitable in cases with marked cervical stenosis or shellfish allergy. Prostaglandins of E series are effective in dilatation and ripening of the cervix, but some of them such as dinoproston require special storage temperatures (2). In recent years, misoprostol (a synthetic Prostaglandin E1 (PGE1)) is widely used in obstetrics and gynecologic conditions such as medical abortion, before dilatation and vacuum aspiration, treatment of post-partum hemorrhage and before hysteroscopy (1, 3-6). Selk et al. published a meta-analysis and systematic review to estimate the benefits and harms of misoprostol usage for cervical dilatation before hysteroscopy (7). They found that there was an increase in side effects among patients treated by misoprostol and concluded that the current evidence does not support the routine use of pre-operative misoprostol before operative hysteroscopy in all patients (7). Some of the adverse effects of misoprostol are nausea, vomiting, diarrhea, vaginal bleeding and uterine cramps (3, 4). Evening Primrose is a plant with yellow flowers which grows wild in North America and some parts of Europe and blooms in the evening. Evening Primrose Oil (EPO) is extracted from the seed of the plant. It is usually commercially available in soft gel form and contains 50-70% linoleic acid, 7-10% gamma-linolenic acid and small amounts of oleic, palmitic and stearic acids (8) (Figure 1). The prostaglandin-1 series, especially PGE1 is anti-inflammatory in nature, decreases cholesterol production and has anti-coagulant and hypotensive effects. Members of PGE2 series, with the exception of thromboxane (PGI2), are inflammatory, and oppose PGE1 (8-10). EPO is used to treat some medical conditions including eczema, premenstrual syndrome, rheumatoid arthritis, diabetic peripheral neuropathy, mastalgia and menopausal symptoms (i e, hot flash) (11). Short-term side effects of EPO may include loose stool and minor gastro intestinal upset (belching, abdominal bloating) (12). An optimal dose of EPO is not established yet. Usually, manufacturers recommend a dose of 3g EPO per day (2 × 500 mg capsules or 1 × 1000 mg capsules three times a day) (13). EPO is traditionally used among Native American pregnant women to ease the cervical priming (14, 15). Ty-Torredes et al. in a randomized clinical trial studied the effect of EPO on pregnant women (16). Capco et al. (17) and Aquino et al. (18) studied the effect of EPO on cervical priming prior to hysteroscopy. Most studies show that synthetic prostaglandins play an important role in cervical ripening, but prostaglandins have some side effects, are not easily available, and are relatively expensive. EPO is a precursor of prostaglandins, especially PGE1 in a readily bioavailable form to the human body. Compared to misoprostol, EPO is an herbal oil with no or a few side effects, is less expensive, more available and easy to use. There are not enough studies to evaluate the efficacy of EPO in cervical ripening before hysteroscopy and only few controversial studies on the effect of EPO on the cervical priming of pregnant women are published (14, 15).
2. Objectives
The current study aimed to determine the effect 1000 mg of EPO on dilatation and the ease of cervical ripening in non-menopausal females without a history of Normal Vaginal Delivery (NVD) and menopausal females who were candidate for operative hysteroscopy.
3. Patients and Methods
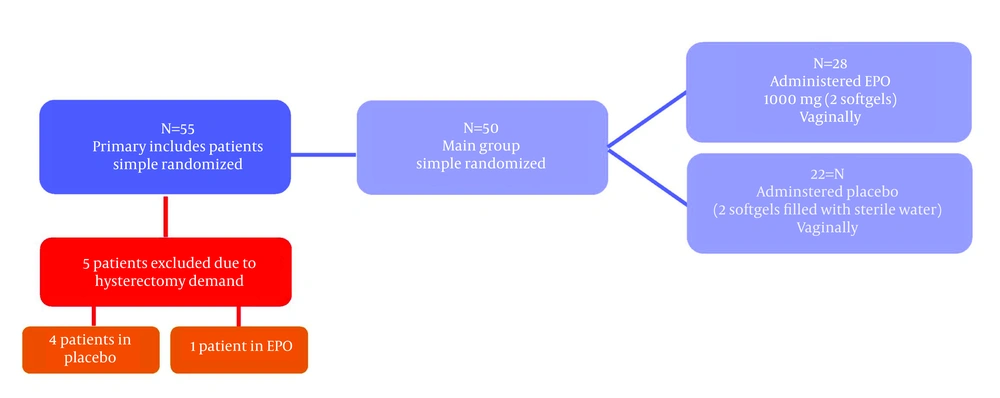
The current study was performed as a randomized double-blind placebo-controlled trial accomplished with a simple coin toss on 50 subjects. The study population was comprised of females between 25 and 75 years referred to Gynecology clinic and were candidates for operative hysteroscopy. The study protocol was approved by the Iran University of Medical Sciences ethic committee. The inclusion criteria were being non-menopausal women without a history of NVD or menopausal women. The exclusion criteria were a history of NVD, contraindications for the use of EPO (including patients with bleeding disorders or consuming medications that inhibit blood clotting, patients with a history of schizophrenia who take phenothiazines and those with epilepsy), structural anomalies of cervix including a history of incompetent cervix, Müllerian anomaly or a history of cervical cone biopsy. Once the subjects were recruited according to the inclusion and exclusion criteria, a general gynecologic examination was performed. Totally, 55 candidates were included after signing a written consent; the subjects were then selected according to a simple randomization (toss of a coin) and divided into two groups. Out of 55 subjects, entering the study five subjects were excluded due to the need for abdominal hysterectomy (four subjects in the placebo group and one in the EPO group). The EPO group received 1000 mg of EPO (Webber Naturals®, WN pharmaceutical Ltd., Canada) in form of two soft gels each containing 500 mg of EPO. The soft gels were placed in the posterior vaginal fornix at least 6 - 8 hours before hysteroscopy in the EPO group and the placebo group received placebo in the same fashion (placebo was EPO soft gel emptied and filled with sterile water). The hysteroscopy was performed under general anesthesia by one surgeon for all patients using a resectoscope (Karl Storz Endoscopy Inc., Germany), with the outer sheath diameter of 11 mm and a 30 -degree forward oblique lens. The primary outcome measures were the time to achieve a 10 mm dilatation of the cervix with Hegar dilators starting with 3 to endpoint of 10 mm as well as the size of the first dilator used to apply force for dilatation of the cervix in each subject. The secondary outcome measures were uterine cervico-vaginal complications and adverse effects related to the use of vaginal EPO. Data analysis was performed using the statistical package for the social sciences (SPSS, Version 11.5 for windows). Kolmogorov-Smirnov test was used to confirm the normality of the quantitative data; as the distribution of the quantitative variable in the study was non-normal, and frequency of menopausal women in both EPO and placebo groups was small, Mann-Whitney U test was used to compare two groups. Chi-square or the Fisher Exact test was used to compare qualitative variables between the two groups. P Values < 0.05 were considered statistically significant.
4. Results
All 50 subjects were randomly divided into two groups; 28 subjects in the EPO group and 22 in the placebo group (Figure 2). There was no statistically significant difference between the two groups regarding the age, baseline cervical dilatation (closed), parity and menopausal viewpoint (Table 1). Seven patients from the EPO group (25%) and seven patients from the placebo group (31.8%) were menopausal women (P = 0.59). Also there was no statistically significant difference between the two groups considering the years after menopause [8.5 (4.75 - 10.50) vs. 5 (1 - 12); P = 0.73]. The median duration of cervical dilatation in EPO group was significantly lower compared to that of the placebo group [33.5 (26.25 - 41.50) sec. vs. 75 (30 - 127.5) sec, P = 0.003]. Also the median size of the first Hegar applied with force in the EPO group was greater than that of the placebo group [8 (8 - 9) vs. 7 (5.75 - 8.25); P = 0.002] (Table 2). Dilatation time and the size of first Hegar applied with force in non-menopausal women are illustrated in Table 2. As it can be observed the dilatation time among non-menopausal women in the EPO group was significantly less than those of the placebo group (P = 0.02). Also the size of the first Hegar applied with force in the EPO group was significantly higher than that of the placebo group (P = 0.007). For menopausal women a similar result was obtained regarding the time. As Table 2 shows, the dilatation time in EPO group was significantly less than those of the placebo group (P = 0.02), but the difference in size of the first Hegar applied with force in EPO and placebo groups was not statistically different among menopausal women (P = 0.06).
| Main Groups (Total) | Non-Menopause | Menopause | |||||||
|---|---|---|---|---|---|---|---|---|---|
| EPO Median (IQR) N=28 | Placebo Median (IQR) N = 22 | P Value | EPO Median (IQR) N = 21 | Placebo Median (IQR) N = 15 | P Value | EPO Median (IQR) N = 7 | Placebo Median (IQR) N = 7 | P Value | |
| Characteristics of Patients | |||||||||
| Age, y | 41 (34.3 - 43.8) | 39 (32.6 - 44.5) | 0.89 | 32 (28 - 39.5) | 27 (24 - 41) | 0.51 | 58 (50.52-61.47) | 56 (47.84 - 60.15) | 0.56 |
| Parity | 2 (1 - 3) | 1 (0 - 2) | 0.26 | 0 (0 - 2) | 1 (0 - 2) | 0.17 | 4 (3 - 7) | 2 (2 - 6) | 0.17 |
| Indications | |||||||||
| AUB, (%) | 21 (75) | 18 (81.8) | 0.73 | 14 (66.7) | 11 (73.2) | 0.72 | 7 (100) | 7 (100) | b |
| Others, (%) c | 7 (25) | 4 (18.2) | 0.73 | 7 (33.3) | 4 (26.7) | 0.72 | 0 | 0 | b |
a Abbreviations: AUB, abnormal uterine bleeding (endometrial hyperplasia, polyp and submucosal myoma); EPO, evening primrose oil; IQR, interquartile range;
b Since all were in one group a P Value could not be calculated.
c Intrauterine adhesions and septum.
| Total | Sub Groups | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Groups | Main Groups | Non-Menopause | Menopause | ||||||
| EPO | Placebo | P Value | EPO | Placebo | P Value | EPO | Placebo | P Value | |
| Variables | Median (IQR) N = 28 | Median (IQR) N = 22 | Median (IQR) N = 21 | Median (IQR) N = 15 | Median (IQR) N = 7 | Median (IQR) N = 7 | |||
| Time, s b | 33.5 (26.25 - 41.50) | 75 (30 - 127.5) | 0.003 | 35 (30 - 43.5) | 60 (30 - 120) | 0.02 | 32.14 (20 - 35) | 100 (30 - 190) | 0.02 |
| Size, mm c | 8 (8 - 9) | 7 (5.75 - 8.25) | 0.002 | 8 (8.06 - 8.79) | 7 (6.35 - 8.04) | 0.007 | 7 (5 - 9) | 10 (7 - 10) | 0.06 |
a Abbreviation: IQR, interquartile range.
b Time to complete dilatation.
c Size at first resistance.
5. Discussion
According to the obtained results, the present study revealed that vaginal EPO can be useful in cervical ripening in terms of the total cervical dilatation time being lower than that of the placebo group (33.5 sec vs. 75 second, P = 0.003), and also the greater cervical width in EPO (the size of the first Hegar applied with force: 8 mm vs. 7 mm, P = 0.002) (Table 2).
Capco et al. in a randomized double-blind, placebo-controlled clinical trial studied the efficacy of EPO on cervical priming among 42 subjects randomly assigned to EPO and placebo groups (17). The primary outcome was the initial cervical dilatation before hysteroscopy. The secondary outcomes were the number of patients who required cervical dilatation and the duration of cervical dilatation to Hegar size of 10mm. There was a difference considering the initial cervical dilatation between the two groups (7.8 mm vs. 4.3 mm). All subjects in the placebo group needed mechanical cervical dilatation, while only 47.6% of the subjects in EPO group required it. The mean time to achieve 10 mm dilation was 17.3 seconds in the EPO group vs. 53.5 seconds in the placebo group (17). It should be noted that in this study some variables such as parity and previous history of NVD, which could influence the results, were not considered. Also, they did not report any uterine, cervico-vaginal complications and drug adverse effects during and after hysteroscopy.
Aquino et al. in a pilot study assessed the effect of EPO on the ease of cervical dilatation before hysteroscopy. Six menopausal and two nulligravid premenopausal patients were candidates for diagnostic hysteroscopy. Two soft gels (2000 mg) of EPO were placed in the posterior vaginal fornix four to six hours before the hysteroscopy. The primary outcome was the ease of cervical dilation to 7 mm to allow diagnostic hysteroscopy by the Likert scale. The secondary objective (secondary outcome of EPO insertion or possible drug reaction) was to determine any cervico-vaginal complications and adverse effects related to the EPO. Preoperative cervical dilation (in mm) after EPO administration was measured by Hegar dilators in an advancing method beginning with a 4 mm Hegar size to the endpoint of 7 mm Hegar size. Adverse outcomes and reactions as well as uterine and cervico-vaginal complications were assessed. All patients who were given EPO achieved a cervical dilation of 7 mm with ease and no adverse effects and uterine or cervico-vaginal complications were reported. They concluded that intravaginal administration of EPO four to six hours before diagnostic hysteroscopy (7 mm diameter) affected the ease of cervical dilatation without any adverse effects (18). In this study, they considered the outcome and adverse effects of EPO, but the sample size was small and they reached only to the endpoint of 7 mm during the dilatation.
The subject study assessed the effect of EPO on cervical ripening among 50 females with similar basic characteristics (age and parity), randomly divided into EPO and placebo groups. With a vaginal dose of 1000 mg EPO, a significantly shorter cervical dilatation time and more initial cervical width were achieved. There were no uterine or cervico-vaginal complications during operative hysteroscopy. Also, adverse effects associated with drug were assessed in the patients in a three -week follow up and no loose stool and gastro intestinal upset were observed in the EPO group. In comparison with the prior studies, the current one used 1000 mg EPO soft gels and target point of 10 mm cervical dilatation in a larger sample size. Overall, EPO is inexpensive, easy to use and seems to be safe, since no complications were reported after its vaginal insertion and during the cervical dilatation.
Block randomization was preferred to the simple one in the current study to prevent groups going uneven. Also, the current study sample size might not be enough in menopause women to assess the effectiveness of EPO on cervical ripening. However, more trials with a larger sample size are necessary to determine the dilatation time and applying the force needed to reach the end point of 10mm dilatation. It is suggested to use a tonometer to record the cervical tone in every step of dilatation in future studies.