1. Background
Hysterectomy is a common gynecological procedure performed as a treatment for both benign and malignant causes. Pelvic masses occurring after hysterectomy can have different origins such as ovarian residues, fallopian tubes, other pelvic organs, and more commonly ovaries. However, there is little information available in this regard (1). Patients with ovarian masses are concerned whether the mass is benign or malignant. Malignant ovarian tumors account for 3% of all gynecological malignancies, with a life-time risk of 1-1.5% and an annual mortality rate of 0.5% (2). Ovarian cancer, as the third most important genital cancer and fifth cause of cancer-related death in women, is diagnosed at terminal stages in 70% of cases. Therefore, it is imperative to identify the possible risk factors of ovarian masses (3). Few previous studies have discussed the histopathological features of ovarian masses detected after hysterectomy. These studies have focused on age, method of hysterectomy, parity, and other causative factors and treatment methods of ovarian cancer (4-6). However, a majority of outpatients visiting gynecological clinics have ovarian masses after hysterectomy, and literature lacks information regarding their prevalence and histopathology.
2. Objectives
We aimed to investigate the five-year prevalence and histopathological distribution of ovarian masses occurring after hysterectomy in Iranian patients, and to determine the need for prophylactic salpingo-oophorectomy and its possible benefits.
3. Patients and Methods
This descriptive cross-sectional study was approved by the ethics committee of Baqiyatallah University of Medical Sciences. For this study, we enrolled outpatients with ovarian masses and a history of hysterectomy for benign conditions (regardless of the method) who were visiting the gynecology clinic of Baqiyatallah Hospital (a government hospital located in Tehran) between May 2009 and May 2014. All study participants were informed of the study protocol and enrolled after they signed the informed consent form. Patients with a positive history or simultaneous malignancy were excluded. Demographic information, pathological features of ovarian masses, family history for ovarian cancer, the time between hysterectomy and ovarian mass surgery, and method of hysterectomy were recorded in a predesigned checklist. The levels of tumor markers, CA125 and alpha-fetoprotein (αFP), were measured in the Baqiyatallah Hospital laboratory. Patients underwent surgical treatment, and the extracted masses were sent for histopathological analysis to a single histopathologist in Baqiyatallah Hospital. The histopathologist was blinded to all personal data of the patients. Data were analyzed using Statistical Package for Social Sciences (SPSS) version 16 (SPSS Inc. Chicago, IL) for Windows. Kolmogorov–Smirnov test was used to test the distribution normality of the data. Statistical frequency was used to determine the prevalence of variables.
4. Results
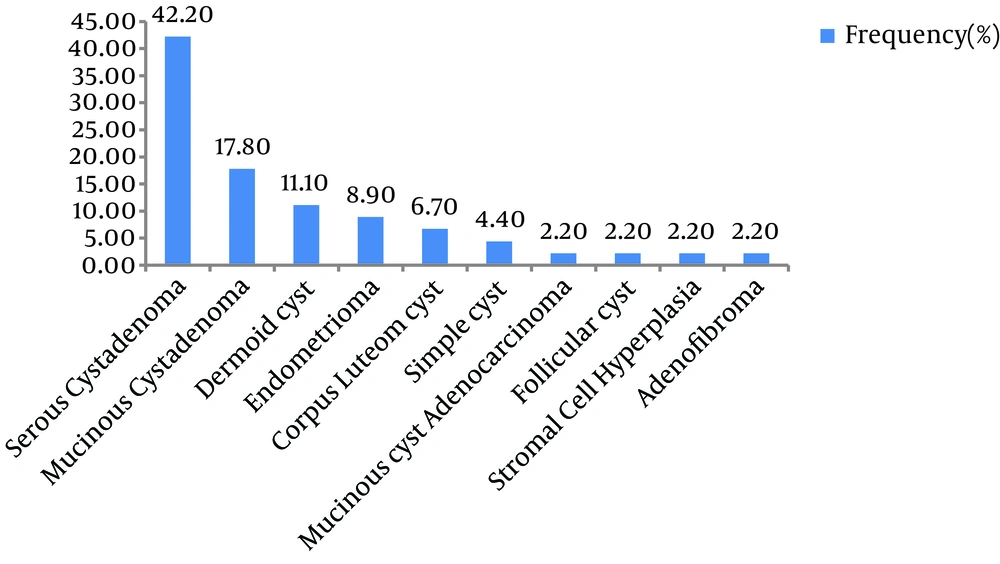
Of the 1052 patients with ovarian masses, the data of 45 (mean age, 53.11 ± 9.56 years; range, 39 - 77 years) patients with a history of abdominal hysterectomy were analyzed. Their mean age at the time of hysterectomy was 47.92 ± 1.58 years (range, 38-68 years). The mean time interval between hysterectomy and diagnosis of the ovarian mass was 5.38 ± 4.15 years (range, 1 - 20 years). The mean CA125 level was 10.53±5.43 (range, 3.5 - 23.4), and the meanαFP level was 2.30±1.08 (range, 0.5-5 years). Based on histopathological reports, serous cystadenoma was the most frequent (43.2%) pathological diagnosis, followed by mucinous cystadenoma (17.5%; Figure 1). Follicular cyst (2.2%), stromal cell hyperplasia (2.2%), and adenofibroma (2.2%) were the less common histopathological diagnoses. Only one case of malignant mucinous cyst adenocarcinoma was reported in a 68-year-old woman with a history of hysterectomy 20 years ago.
5. Discussion
Histopathological evaluation of the ovarian masses in the present study revealed that serous cystadenoma was the most frequent histopathological diagnosis among ovarian masses, followed by mucinous cystadenoma. The less common ones included follicular cyst, stromal cell hyperplasia, and adenofibroma. In an observational analytical study by Bhatnagar et al., the mean age for the incidence of ovarian mass after hysterectomy was reported to be 42.3 years in Pakistani patients (1); this age is significantly lower when compared with the results of the present study. This could be because of the minimum age of patients in their study was 27 years. In a retrospective study assessing ovarian cancer after hysterectomy among American patients, McGowan reported 18 years as the mean time interval between abdominal hysterectomy and the diagnosis of ovarian cancer; the corresponding time was 10 years for the vaginal hysterectomy group (4). In the present study, all the benign masses were diagnosed one to ten years after hysterectomy, and the only malignant case was diagnosed 20 years after hysterectomy; this finding suggests that ovarian masses diagnosed sooner after hysterectomy are more likely to be benign. In agreement with this result, Loft et al. reported that the protective effect of hysterectomy on the incidence of ovarian cancer decreases over time in Danish women (5). In a prospective study by Plockinger et al., study participants with ovarian mass had a mean age of 43.9 years at the time of hysterectomy, which is significantly lower in comparison with the age noted in the present study. Patients with no ovarian masses had a mean age of 39.3 years at the time of hysterectomy (7), and at a 10-year follow-up after hysterectomy, no ovarian malignancy was recorded. Tumor markers, CA125 (less than 35 U/mL) and αFP (less than 40 ng/mL), were found to be in normal ranges in all study participants, indicating that most ovarian masses occurring after hysterectomy are benign. Holub et al. in a prospective study reported an incidence rate of 5.67% for ovarian masses after abdominal hysterectomy, 3.8% after laparoscopic hysterectomy, and 0.67% after vaginal hysterectomy (6). In a prospective study by Chan et al. patients who underwent hysterectomy with bilateral, unilateral, and no salpingo-oophorectomy were followed up for the incidence of ovarian cancer (8). The rate of ovarian cancer was 26.2 for those with hysterectomy alone, which comprised most of the population. Therefore, Chan et al. concluded that removal of both ovaries decreases the incidence of ovarian cancers (8). On the other hand, Chiu et al. reported the case of a 52-year-old woman with pelvic mass and high serum levels of CA125 and CA199 and a history of hysterectomy and bilateral salpingo-oophorectomy for uterine myomas and endometriosis 13 years ago. The pelvic mass was an ovarian serous tumor derived from remnant ovarian tissue; this suggests the importance of surgery and surgeon's skill (9). Vorwergk et al., evaluating the benefit of prophylactic bilateral salpingo-oophorectomy in standard hysterectomy, concluded that bilateral salpingo-oophorectomy reduced the occurrence of adnexal pathologies, which could also require re-intervention (10). Our findings suggest that a majority of ovarian masses, especially those diagnosed in a shorter time interval after hysterectomy, are benign and that the protective effect of hysterectomy on the incidence of ovarian cancers decreases over time. Thus, it can be concluded that Iranian patients with asymptomatic ovarian masses that are diagnosed sooner after hysterectomy and are associated with negative tumor markers could be followed up, and prophylactic oophorectomy may not be necessary. Further prospective and long-term follow-up studies with a larger sample size are needed to evaluate all plausible risk factors.