1. Background
Simplifying the therapeutic regimen of liver transplant recipients, could be a support to the avoidance of severe rejection and graft failure (1). With appropriate planning, liver recipients could have improved opportunities related to the quality and quantity of life (QQL), similar to normal healthy control subjects, without significant biochemical laboratory changes in liver and renal function (2). Currently, liver transplantation is an established treatment for patients with end-stage liver diseases (ESLD) in Iran. Through available records related to liver transplantations, there has been significant improvement in the development of immunosuppressive agents such as tacrolimus (3). Tacrolimus has a variable and low bioavailability (F), high volume of distribution (Vd), extensive hepatic metabolism, that mostly excreted into the bile. The metabolism of this drug is catalyzed by cytochrome P450 3A (CYP3A) and is susceptible to modulation by CYP3A inducers and inhibitors. The therapeutic blood tacrolimus level is recommended to be from 5 - 15 ng/mL (3-12). Tacrolimus inhibits thymocyte differentiation, damages thymic epithelial cells, and prevents apoptosis of antigen and mitogen stimulated T-cells. According to previous publications, neurotoxicity, akinetic mutism, nephrotoxicity, new onset diabetes (after transplant), gastro-intestinal toxicity, hepatotoxicity, and thrombotic microangiopathy could be linked to high levels of tacrolimus after liver transplantation (3-20).
Neurotoxicity associated with tacrolimus may start at therapeutic concentrations, as vasogenic edema (reversible) and cytotoxic edema (irreversible) That could cause changes in the subcortical white matter and posterior cerebral artery. Posterior reversible encephalopathy syndrome is the primary and rapid result of calcineurin inhibitor neurotoxicity. The characteristic neurological patterns related to the recipients of organ transplantation could be described as: altered mental status, headache, visual turbulences, and seizures (15-20). Pharmacotherapy management of tacrolimus early in the post-transplantation period needs well-focused attention, regarding the monitoring of drug concentration (3, 21, 22). In order to create more long-term survivors and excellent outcomes, the number of studies and articles related to tacrolimus therapy in the Iranian liver transplant population are growing. Neurological complications are not unusual and could contribute to a longer intensive care unit and hospital stay. Therefore, every attempt should be made towards early detection of immunosuppressive therapy-related adverse effects.
2. Objectives
The aim of this study was to investigate the evidence-based pharmacotherapy of Prograf levels that might be related to neurotoxic borders in liver transplant recipients.
3. Patients and Methods
This study was conducted at the Isfahan neurosciences research centre. A preliminary cross-sectional study of fifty liver transplant recipients comprised of 16 females and 34 males with a median age of 49 years (range: 25 - 64 years) was performed. Furthermore, an evidence-based Prograf therapy treatment, linked to studies of complications, was performed in a retrospective manner by reviewing recipients’ medical records. Trough levels of tacrolimus were determined using the microparticle enzyme-mediated immunoassay technique. The patients’ clinical, pharmacological, and demographic data were recorded in Excel and statistical analysis of the variables was performed using SPSS for Windows. This study was approved by the ethical committee of Isfahan neuroscience research centre and Isfahan deputy of research, with the code number of 291224.
4. Results
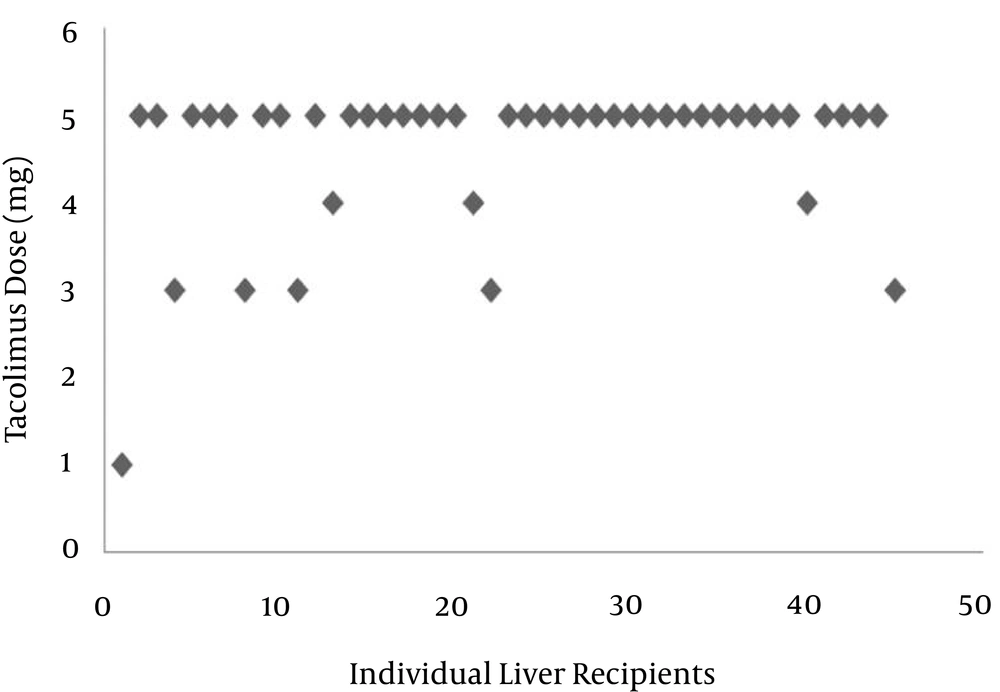
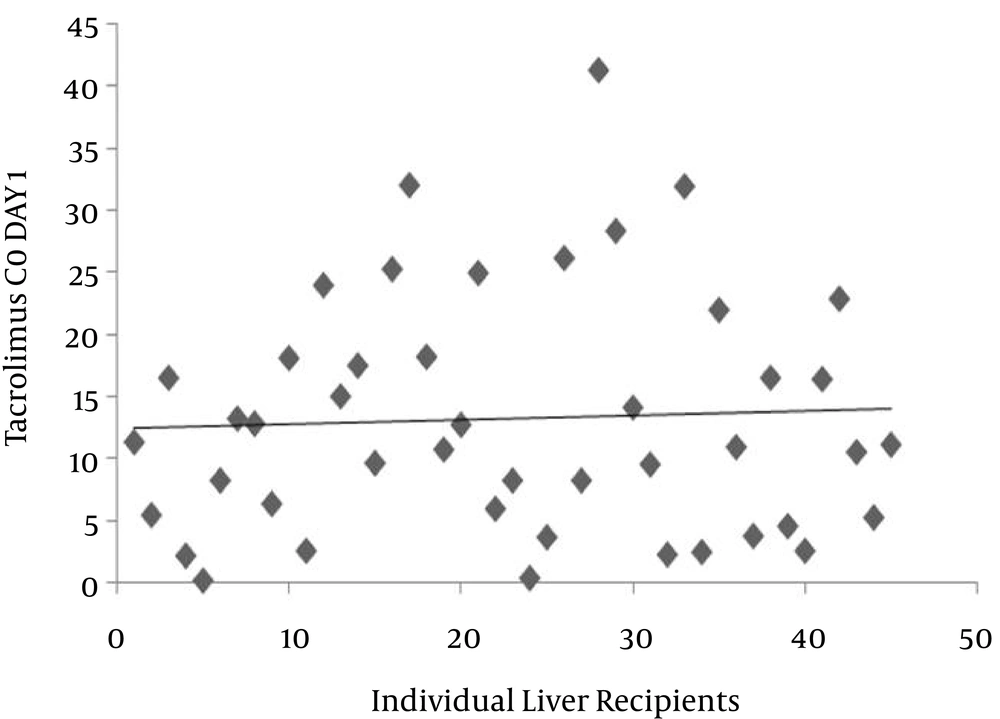
The distribution of immunosuppressive trough levels in the population studied was highly variable. As shown in Figure 1, the mean dosage of the drug was 4.6 mg (range: 1 - 5 mg). Out of 45 patients, the doses of tacrolimus in 80% were 5 mg. Because the management of tacrolimus was connected with marked unpredictability and a large disparity in C0, thus, the efficiency and neurotoxicity seem to be associated with blood levels. As shown in Figure 2, the mean C0 of tacrolimus was 13.2 mg/L, with a minimum value of 0.1 and a maximum value of 41.4 mg/L. Trough levels of tacrolimus in 20.4% of patients was lower than the suggested therapeutic level. In 35% of the patients studied, the level was more than 15 mg/L (neurotoxic border). There was no correlation between dose and trough levels (P = 0.270, r = 0.168). The pattern of pharmacotherapy for selected patients is described as follows (Box 1): A twenty-four-year-old male with code 45, due to autoimmune hepatitis, received prednisone, and azathioprine eight years prior to liver transplantation. After surgery, the patient presented with a herpetic lesion on his lip that had been treated with intravenous acyclovir followed by the oral form. The drug regimen after transplantation was as follows: Prograf 3 mg every 12 hours, tablet; CellCept 1 gm every 12 hours, capsule; fluconazole 100 mg every 12 hours, tablet; co-trimoxazole 1 g daily, tablet; folic acid 5 mg 1 daily, tablet; prednisone 20 mg every morning, tablet; pantoprazole 40 mg every 12 hours, tablet; calcium D every 8 hours, tablet; and acyclovir every 12 hours. Tacrolimus C0 was reported as 6 mg/mL. In another twenty-eight-year-old male transplanted due to primary sclerosing colangitis (PSC) with code 89, the drug regimen was as follows: amp cefitizidime 1 gm; metronidazol 500 gm every 6 hours; pantoprazole 40 gm every 12 hours; methylprednisolone 1 gm a day after transplant for two days; heparin 10000 iu for 24 hours; and mesalasin-albumin 1 vial. Evidence of acute rejection was noted in the patient’s medical record, and then the patient was treated with 3 gm methylprednisolone. The patient was discharged with the following drug regimen: tacrolimus 7 mg; CellCept 2 gm; and prednisone 20 mg. Tacrolimus C0 was reported as 5 mg/mL. Another patient, with code 68, underwent liver transplantation due to Wilson’s disease. After the operation, the patient showed psychosis and episodes of rejection. The drug regimen was as follows: Prograf 4 mg every 12 hours; CellCept 2 gm every 12 hours; and prednisone 20 mg every 12 hours.
| Types of Drugs Prescribed in Each Individual; Expected Behaviors in Combination Therapy (1-32) |
|---|
| Prograf, CellCept, fluconazole, co-trimoxazole, tab prednisolone pantoprazole, acyclovir; poly pharmacy could increase the CNS toxicity of tacrolimus , the risk of rejection still remains challenging and is; tacrolimus and CellCept, both may increase CellCept levels, risk of toxicity, may increase risk of immunosuppression, skin and other malignancies, infections, sepsis (renal excretion decrease by nephrotoxic agents; additive effects); co-administration of pantoprazole (proton pump inhibitors PPIs with CellCept) has been reported to reduce the exposure to mycophenolic acid, therefore, the risk of rejection still remains challenging and is; a fairly significant interaction exists between co-trimoxazole and CellCept, therefore, combinations usually should be avoided; sulfamethoxazole may decrease the blood levels and effects of mycophenolic acid |
| Cefitizidim, metronidazole, pantoprazole, methylprednisolone;co-administration of tacrolimus with metronidazole may result in elevated tacrolimus concentrations, possibly leading to tacrolimus toxicity |
| Citalopram, pantoprazole, co-trimoxazoe, CellCept, Prograf, prednisolone, siroloimus;citalopram and tacrolimus both, may increase risk of QT continuation, cardiac arrhythmias (additive effects); using tacrolimus together with prednisone may increase the blood levels and effect one or both medications |
5. Discussion
Streptomyces fermentation product tacrolimus belongs to group of calcineurin inhibitors. It is an extensively used immunosuppressive drug for avoiding rejection associated with organ transplantation (23). Previous publications have suggested that compared to cyclosporine, tacrolimus has superior immunosuppressive effectiveness and a lower frequency of adverse effects. In liver transplantation, the drug has been established as a first-line treatment (3-7, 24). The transcriptional factor, nuclear factor-kappa B (NF-κB), is involved in both neuronal cell death and survival. Studies have showed that chronic administration of this drug considerably decreases spontaneous optic neuropathy in p50-deficient mice (25).
The wide range of values for trough levels of tacrolimus observed in this study is in agreement with previous publications (3, 26, 27).
Previous publications have confirmed that about 10 - 28% of patients with calcineurin inhibitors (CNIs), such as tacrolimus, develop some type of neurotoxicity. This includes tremors, paraesthesias, and acute polyneuropathy that are more widespread with tacrolimus than cyclosporine. The pathogenesis may be similar to that of central white matter lesions, where injury to both the major and minor vasculature may cause hypoperfusion or ischemia, and local secondary toxicity in the white matter (28). A recent publication confirms that the donor livers’ CYP3A-status, taking both CYP3A5 allelic variations and CYP3A4 expression into account, can better identify the risk of CNI overexposure or underexposure, and may contribute to the avoidance of misdosing-induced graft injury in the early postoperative period (29).
Immunosuppressive therapy, joined to polypharmacy, could cause destructive consequences due to irregular metabolism of tacrolimus by hepatic enzymes in liver transplant recipients. Drugs, such as proton pump inhibitors and some antiepileptic drugs (AEDs), could increase trough levels (C0) of tacrolimus by inhibiting metabolism of the drug. In 35% of the population studied in this research, tacrolimus levels higher than 15 mg/L seemed to be on the neurotoxic side. High levels of tacrolimus might be associated with nephrotoxicity and neurotoxicity. Previously published articles have suggested a significant correlation between drug levels and the incidence of neurotoxicity and nephrotoxicity. Nervous system disorders have been reported as: tremors, headache and insomnia, paraesthesia, and seizures (3, 15-32).
Analysis of the data presented in this work confirmed heterogeneity between individual Prograf levels and an irregularity in the correlation with dosage administration in the Iranian population. Finally, to prevent side effects therapeutic drug monitoring of tacrolimus, and consequently dosage adjustment based on level measurement, is suggested to be valuable.