1. Background
Polycyclic Aromatic Hydrocarbons (PAHs) are of the major environmental contaminants of soil, water and atmosphere which are procuded by human and also natural processes (1, 2). Forest fire, volcanic eruption, natural and fuel combustion, industrial processes and oil refining are the major sources of PAHs production (3). New research studies have focused on th PAHs toxicity (4); PAHs are chemically lypophilic and accumulate in soil sediments and living organisms (5). One of the most important types of PAHs is Phenanthrene with low molecular weight. The half-life of Phenanthrene is approximately 16-126 days in soil sediments (6). Organic contaminations, especially PAHs, are more than standard values in petrochemical and refinery wastewaters (7); these PAHs contaminations are spreading in the surrounding area of these industrial locations. Phytoremedition has several advantages as the removal method of choice in the mentioned areas. Phytoremediation is a cheap soil treatment method and is effective on all oil pollutants especially on toxic pollutants like heavy metals, Polychlorinated Biphenyls (PCBs) and PAHs (4). Salicorniaeuropea, an annual halophyte plant of Salicornioidae family, is grown in coastal areas and plant banks. These are variations of Salicornia species. They are indigenous in Europe, United stated of America and Iran (8, 9). Salicorniaeuropea is able to grow in the salty soil areas of Iran, like Eshtehard’s river banks (“Shur” River),. It is a short plant with less than 30 cm height with some subordinate (auxiliary) stalks in some varieties. Some species have small leaves and some others are leafless. Salicornia species are usually green (10). They can be eaten raw or cooked. And also could be used as domestic animal food (11). Salicornia species can uptake Phenanthrene, Pyrene and Crysene more than other PAHs (12). In a research in Argentina, the biological impact of natural ultraviolet radiation between 290 and 320 nm (UVB), on Salicorniaambigua demonstrated that Salicornia appears to be a good indicator to assess the impact of Ozone depletion on austral ecosystems (13). In these methods, microbiological treatments were used instead of costly physicochemical methods of contaminant remediation, especially industrial contaminants (14).There are a variety of these methods available(15), most reported method are the bacterial (16, 17) and fungal (18) bioremediation but several studies also describe the plant bioremediation, alternatively called phytoremediation (19). Phytoremediation is a technique in which physiologic interactions of plants and organisms lead to absorption and accumulation of organic and inorganic contaminants in soil, water and atmosphere (20). Specific mechanisms of plant phytoremediation which is related to the contaminated environment and contaminants’ characteristics have been investigated (4). PAHs can be efficiently absorbed by phytoremediation methods. Plants absorb thePAHs through different mechanisms. Highly lypophilic characteristics of PAHs mediates their absorption by root epidermis in soil (8).
2. Objectives
In this study, the quantity of Salicorniaeuropiea bioremediation (phytoremediation) on contaminated soil by crude oil was investigated and Phenanthrene was chosen as the representative substance indicating PAHs contamination.
3. Material and Methods
3.1. Plants Materials
Salicornia seedlings were taken from “Shur” River bank which is placed in Rahmanieh village, near Eshtehard area which is within the fifteen kilometers of Karaj, Alborz, Iran. Thirty two seedlings with the same size, age and length were transplanted in 2 kg loam (clay, silt and sand) pots and were cultivated at the depth of 0.5 cm. They had a length of 1.5 cm at the time of planting. Two of them were planted in each pot. They were nourished by 200 ml Hoagland and 50 mmol NaCl, daily (Hoagland is an adequate supplement for plant growth) (21). Average temperature was 20 °C in mornings and 30 °C in afternoons and the experiment took place in the summer. Seedlings were maintained in the same condition for one week.
3.2. Pollutant Exposure Experiment
Thirty two samples in were cultivated 16 pots, divided into one control pot and 15 experimental pots. Fifteen experimental pots were divided into five experimental groups and received five different concentrations of crude oil as contaminants. Five experimental groups were exposed to pollutants within the first two weeks of July.
Two kilograms of loam (clay, silt, sand) were weighed on a digital scale and allocated for each pot. Crude oil concentrations were mixed with weighed loam (soil) in a metallic container completely. To prevent the crude oil to be washed away, soil was shaped into a pile and crude oil was gradually poured on soil. After crude oil and water absorption by soil, the soil was mixed and then translocated into pots. After crude oil inoculation to the soil, two plants which had a length of 15 cm on average were transplanted in each pot. During the period of this study (2 weeks), plants were nourished by Hoag land and 50 mmolNaCl daily. Crude oil and pigments were seen on the top of plants, on the first two days of the experiment.
3.3. Plant Growth and Physiological Measurement
During our experiment, greenhouse temperature was measured by thermometer twice a day (at10 am and 4 pm). Greenhouse condition was modeled as natural sun beam during the day (which is the condition in Karaj). We tied up a piece of string at the base of the stalk which was out of the soil and lengths of stalks were measured from the string to the top of the longest stalk. Also we tied up a piece of string around the middle of stalk for the measurement of plant stalk circumference. Quantitative analysis was performed for color and freshness after two weeks, at the end of the experiment. Four quantitative levels were chosen as standard and plants were scored on their basis.
3.3.1. Levels
Completely green/ natural firmness/tangible moisture
One third of plant was yellow/ intangible moisture
Two third of plant was yellow/ subordinate (auxiliary) stalks were withered/ intangible moisture
completely yellow/died and lignified plant / intangible moisture
At the end of the study, after last measurement of quantitative indicators, plants were removed from soil. Soil around roots were completely cleaned and returned back to its pot. Plants were put into aluminum foil separately, the air in the packs was completely emptied. The same procedure was done for soil of each pot and then all samples of soil and plants were put in a 4 °C refrigerator. Furthermore, five grams of plants’ stalks were cut and put in the oven. After five minutes they were taken out and weighed frequently till samples’ weights were fixed. In this way, sample moisture was measured.
3.4. Chemical Analysis For The Sample of Plant
Extraction was obtained from three grams of plant tissue (mixture of each three repeated samples of each concentration). Plant tissues (stalks and roots) were cut into small pieces, then 60 ml of solvent (chloroform: petroleum ether (1:1)) were added. The suspension was maintained at room temperature for 24 hours for extracting organic contaminants. The mixtures were filtered and Thin Layer Chromatography (TLC) was used for the identification of phenanthrene with petroleum ether as the mobile phase. The solvent was evaporated and purification was carried out by column chromatography with silica as the stationary phase and 300 ml of petroleum ether as mobile phase. The solvent was evaporated under reduced pressure and was dissolved in 10 ml of petroleum ether. The ultraviolet (UV) absorbance at 286 nm was measured by UV Spectrophotometer (UV detector CECIL CE 1021).
3.5. Chemical Analysis for the Sample of Soil
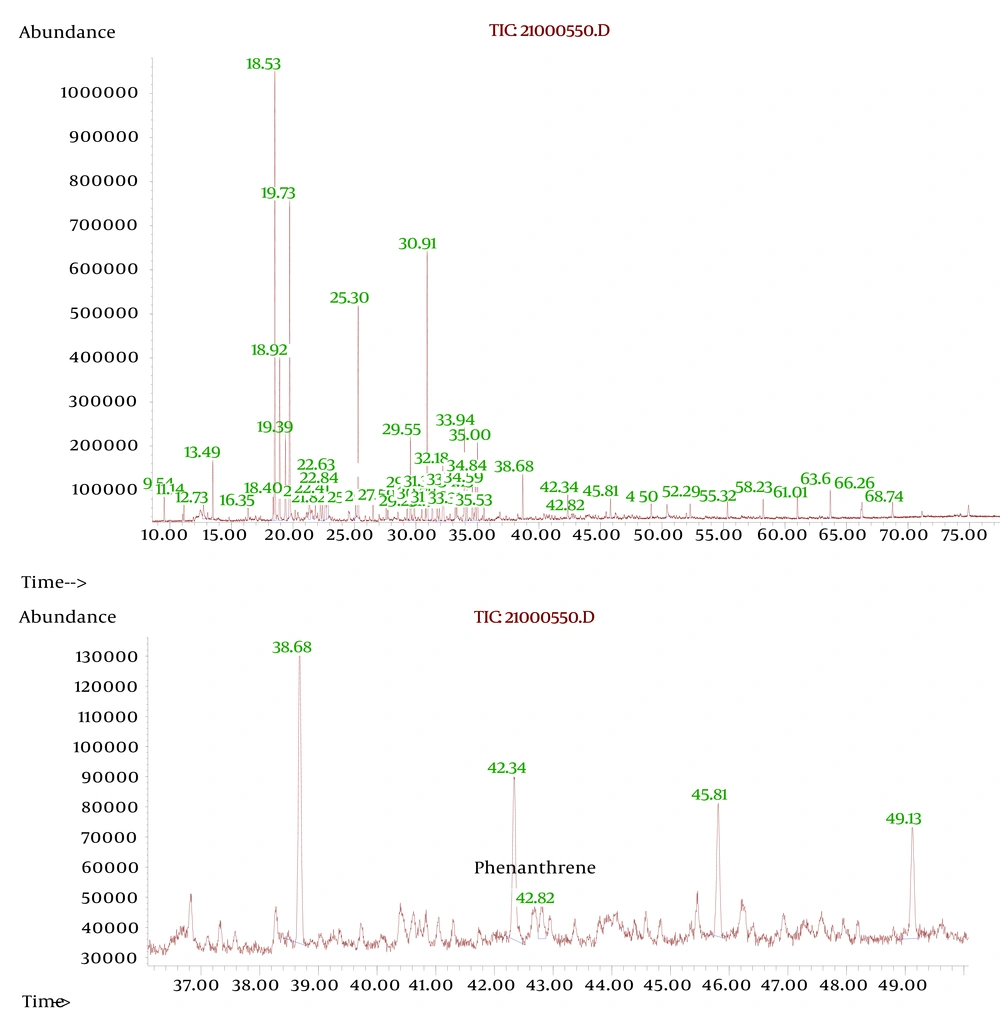
Extraction was obtained from 15 g of soil samples (mixture of each 3 repeated sample of each concentration). Each samples of soil were placed in a 200 ml bottle and 100 ml of chloroform was added to each ones: petroleum ether (1:1) was added. This suspension was maintained overnight for extracting organic contaminants. The mixtures were then filtered and extra liquid evaporated. Purification was carried out by column chromatography with silica as the stationary phase and 300 ml of petroleum ether as mobile phase; the solvent was evaporated under reduced pressure and was dissolved to 10 ml of petroleum ether. Then, one mL of this solution was diluted to 50 ml within petroleum ether (diluted to 1/500). The UV absorbance at 286 nm was measured by UV Spectrophotometer. The extractions from plant sample number four was checked out to confirm the presence of phenanthrene by plants, using Gas chromatographic–mass spectrometric (GC-MS). A retention time of 42.34 minute was observed for phenanthrene with a complete matching fragmentation between extraction from plant and standard phenanthrene solution.
3.6. Statistical Analysis
Data are expressed as mean of two samples of each pot. The Statistical Package of Social Science version 16.0 (SPSS, Chicago, Illinois, USA) was used for data analysis. Statistical significance was noted for P ≤ 0.05. For comparison between the height, humidity and circumference of two groups, independent-samples T-Test was used. Mantel Hansel test (Odds ratio: ORMH) was used to eliminate the confounding factors.
4. Results
Appearance of plants in six experimental groups is reported in Table 1. Stalks’ height and circumference of stalks (Table 2) and humidity of plants’ stalk (Table 3) are illustrated in Tables 2 and 3, and respectively. Moisture and circumference of plants’ stalks did not have statatistically significant difference (P = 0.13 and P = 0.09 respectively), plants height which were 14 to 15 cm on average, did not change significantly (P = 0.45) but high contaminant concentration was associated with decreased plants’ height (Table 2).
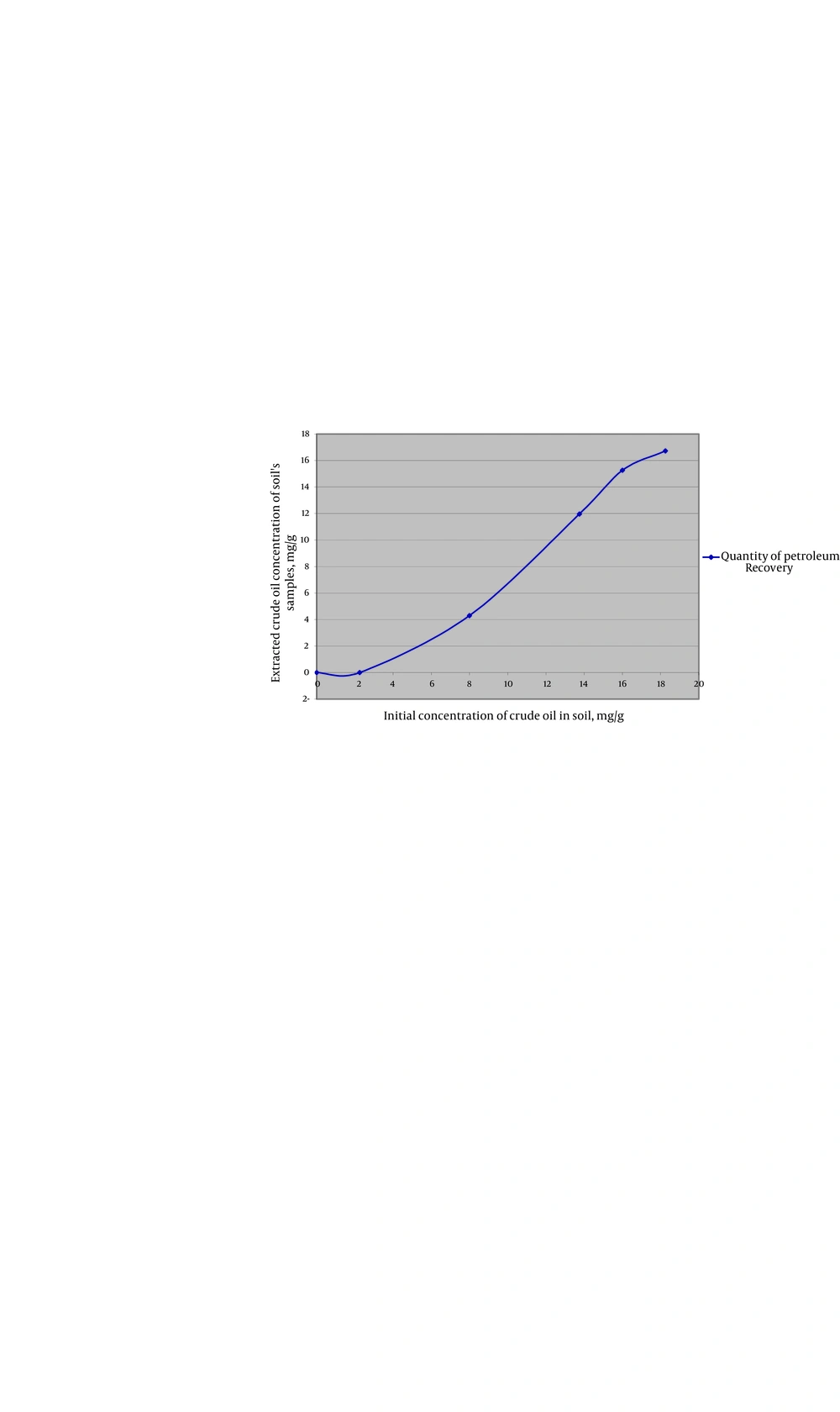
Plants were completely able to uptake crude oil under 2/25 mg crude oil per 1 g of soil and maximum rate of contamination (PAHs) uptake by plant was 6/92 mg from 8 mg (86.5%) crude oil per 1 g of soil (Table 4). Results of UV spectroscopy method are illustrated in Table-5(UV absorbance of soil and plant samples were measured for each sample, repeated ones and the control). Mean UV absorbance in each concentration and also mean UV absorbance of each control is listed. UV absorbance of samples of plants confirms the initial crude oil concentration which was added to pots. By increasing the amount of initial contaminants in soil, UV absorbance of soil also goes up (Table 4).
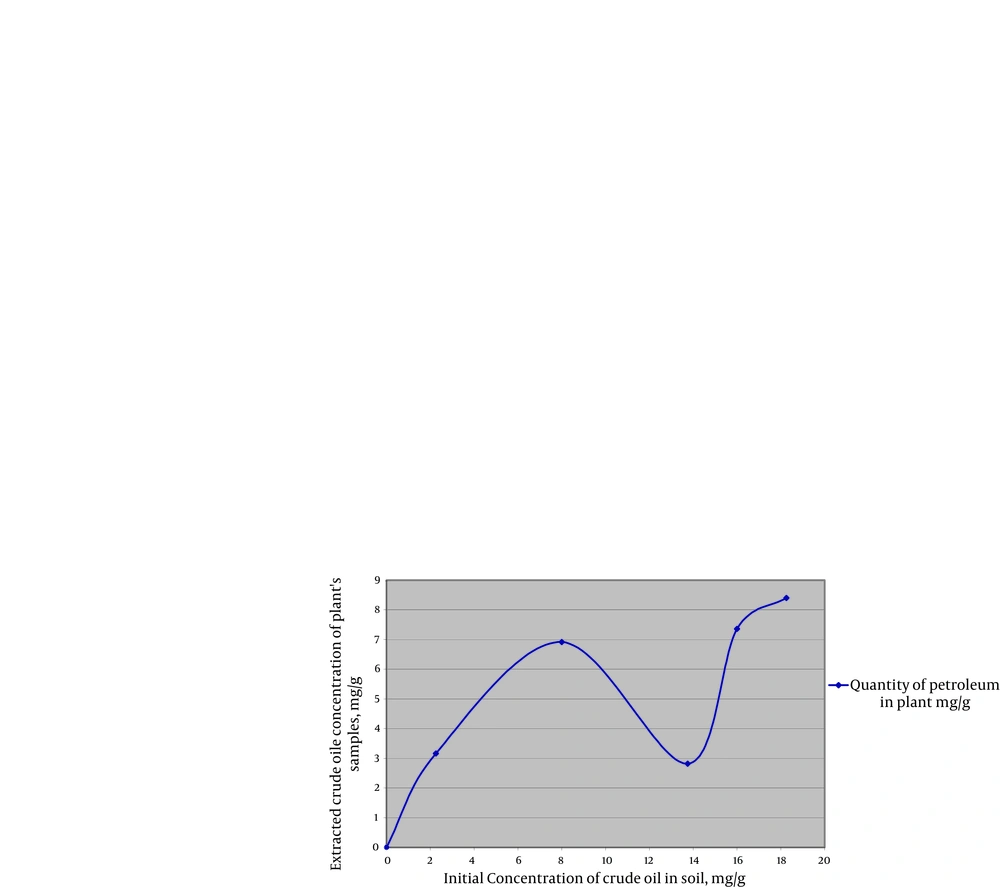
By increasing the amount of contaminants (in high contaminants’ concentration) more than 8 mg crude oil per 1 gram of soil, the absorbance of contaminants (PAHs) by plant samples was reduced (Figure 1). At the concentration of 13/75 mg crude oil per 1 g of soil, samples showed the least uptake of PAHs. When contaminant’s concentration went over 13/75 mg per 1 g of soil, again more amount of PAHs was absorbed by plants which seems that might be due to the compatibility of plants’ defense mechanism with contamination (Figure 1 and 2). and By increasing the amount of contaminants (at highest contaminants’ concentration) more than 8 mg crude oil per 1 gram of soil, the absorbance of contaminants (PAHs) by the samples was reduced (Figure 1). TLC demonstrated that crude oil which we used in this experience contained Phenanthrene. The result was verified by GC-MS test which demonstrated Phenanthrene peak at RT42/34 minute (Figure 3).
| Samples | Height | Circumference | ||||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T1 | T2 | T3 | T4 | |
| Control | 15.1 | 15.1 | 15.2 | 15.25 | 1.1 | 1.1 | 1.09 | 1.09 |
| 1-1 | 14.5 | 14.3 | 14.3 | 14.2 | 1 | 0.95 | 0.95 | 0.95 |
| 1-2 | 15.3 | 15 | 15.1 | 15.1 | 1.2 | 1.14 | 1.15 | 1.1 |
| 1-3 | 15 | 14.9 | 14.7 | 14.6 | 1.2 | 1.18 | 1.2 | 1.2 |
| 2-1 | 15.2 | 15 | 15.1 | 15 | 0.9 | 0.9 | 0.9 | 0.9 |
| 2-2 | 14.5 | 14.5 | 14.5 | 14.4 | 1.3 | 1.26 | 1.26 | 1.23 |
| 2-3 | 15.2 | 15.2 | 15.1 | 15 | 1.1 | 1.1 | 1.1 | 1.1 |
| 3-1 | 15 | 15.1 | 15.1 | 15.1 | 1.1 | 1.1 | 1.1 | 1.15 |
| 3-2 | 15 | 14.8 | 14.9 | 14.9 | 1 | 1 | 1 | 1 |
| 3-3 | 15.5 | 15.6 | 15.6 | 15.5 | 1 | 0.93 | 0.94 | 0.94 |
| 4-1 | 14.6 | 14.4 | 14.3 | 14.3 | 1 | 0.95 | 0.94 | 0.94 |
| 4-2 | 15 | 14.9 | 14.8 | 14.8 | 0.9 | 0.9 | ND | ND |
| 4-3 | 15 | 15 | ND | ND | 1.1 | 1.1 | 1.02 | 0.91 |
| 5-1 | 15.3 | 15.1 | 15 | 14.8 | 1 | 1 | 0.9 | 0.89 |
| 5-2 | 15.5 | 15.2 | 15 | 14.9 | 1.1 | 1.1 | 1.05 | 0.9 |
| 5-3 | 15.7 | 15.6 | 15.4 | 15.2 | 1 | 1 | 1.07 | 1.07 |
a Abbreviations: ND, non-detectable.
| Sample | Primary Weight | Last Weight | Difference |
|---|---|---|---|
| 1-1 | 44.47 | 33.15 | 11.32 |
| 1-2 | 42.13 | 30.36 | 11.77 |
| 1-3 | 43.05 | 32.89 | 10.16 |
| 2-1 | 46.15 | 32.87 | 13.37 |
| 2-2 | 43.37 | 37.6 | 5.77 |
| 2-3 | 45.83 | 32.31 | 13.52 |
| 3-1 | 42.68 | 37.67 | 5.01 |
| 3-2 | 40.89 | 36.85 | 4.04 |
| 3-3 | 44.65 | 38.89 | 5.76 |
| 4-1 | 44.02 | 35.38 | 8.64 |
| 4-2 | 40.32 | 33.34 | 6.98 |
| 4-3 | 39.88 | 33.52 | 6.36 |
| 5-1 | 38.68 | 35.21 | 3.47 |
| 5-2 | 37.77 | 34.38 | 3.39 |
| 5-3 | 38.98 | 33.93 | 5.05 |
| Control | 51.71 | 40.06 | 11.65 |
| Groups | Concentration Petroleum, mg/g | UV Absorbance of Soil, % | Quantity of Petroleum Recovery, mg/g |
|---|---|---|---|
| Control | 0 | 0 | 0 |
| Sample 1 | 2.25 | 20.26 | 3.16 |
| Sample 2 | 8 | 44.36 | 6.92 |
| Sample 3 | 13.75 | 18.09 | 2.82 |
| Sample 4 | 16 | 47.13 | 7.36 |
| Sample 5 | 18.25 | 53.80 | 8.4 |
| Groups | Control | Soil a | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 |
|---|---|---|---|---|---|---|---|
| Repeat1 | 0.162 | 0.242 | 0.162 | 0.438 | 0.916 | 1.142 | 1.222 |
| Repeat2 | 0.18 | 0.242 | 0.18 | 0.444 | 0.935 | 1.15 | 1.242 |
| Repeat3 | 0.153 | 0.293 | 0.153 | 0.438 | 0.942 | 1.134 | 1.246 |
| Mean | 0.165 | 0.259 | 0.165 | 0.44 | 0.931 | 1.142 | 1.236667 |
| Mean-control | 0 | 0.094 | 0 | 0.275 | 0.766 | 0.977 | 1.071 |
aUV absorbance checked for soil without contamination and plant
5. Discussion
Phytoremediation method and using plants as contamination indicator have become a common practice, this is a cost-effective method and it can be used as ground cover in the polluted areas (22).
Our study determined the quantity of crude oil contamination uptake by Salicorniaeuropea roots and stalks (phenanthrene was as an indicator of PAHs in this study). It seems that the heavy crude oil which was used in our experiment caused the fast bioaccumulation of contaminants which led to formation f of black spots (pigmented contaminants) on the plant stalks on the first two days of experiment. In much higher concentrations of crude oil, plants were not able to survive which seems to be the result of physical characteristics of crude oil which causes damage to plants tissues (Table 5) and appearance of the plants confirms our result. At lower concentrations of crude oil, plants had a better appearance; on the other hand at high concentrations of the crude oil, they did not survive (Table 5).
Meudec et al. proved the fast bioaccumulation of PAHs from oil polluted sediments in roots of halophytic plant, Salicornia fragilis which confirms our result (8). Salicornia species are efficient indicators for different kinds of pollutants. In a recent study, the effect of mercury (Hg)on environment has been assessed and also in this study Salicornia species which were the main ingredient of cow’s food , were involved in the contamination (11).In an extensive research by Rosso et al. cadmium (Cd) and vanadium (V) uptake ( two kinds of heavy metals) by Salicornia virginica were investigated and this study showed that Salicornia species can effectively accumulate heavy metals such asHg, Cd and V (21). In another study in United States, biological volatilization of selenium in Salicornia bigelovil (pickle weed) was found to the greatest extent (23).In another recent research in France, Salicorniaeuropea was used to solve the problem of human liquid wastes in biodegenerative life support system (BLSS) and they showed that types of nitrogen they used did not influence the Salicorniaeuropea appearance;in a similar manner we used this plant for absorbing the low concentrations of crude oil as contaminant (At 6/92 mg/g, the best result) (24).
A.Meudec et al. introduced GC-MS as a novel method of determination of PAHs in plants, which corresponds to our identification method (12). In our study, spectrophotometery in the range of ultraviolet C light (UVC: 220 to 290 nm wavelength in which PAHs have a considerable absorbance) was conducted and the quantitative data correlated with the amount of crude oil absorbance and maximum level of tolerance in plants (plant number 4 showed the maximum level of tolerance). Also Meudec et al. investigated the bioaccumulation of PAHs in the shoots by GC-MS in plants and sediments (8). It seems that Salicorniaeuropea survives well at high concentrations of crude oil contamination (18.25 mg crude oil in one g of soil) and we found that not only PAHs compounds can be reason for this effect but also physical characteristics of crude oil can be a responsible factor. A previous study, which showed physical properties of fuel contributes to damage to plant tissue, is in accordance with our results, where they also suggested that the reflectance of plant tissue can be regarded as an indicator of plants’ stress level (25). The results of the present study demonstrated that increased amount of contamination form 8mg crude oil per 1 g of soil, enhances the efficiency of plant while physical damage will not be t observed. Our results also showed that the adaptation and capacity of plant for bioremediation is noticeable. Increasing concentration of contaminants more than 16 mg/g which caused physical damages prevented plant uptake mechanisms. Based on the results of this study, Salicorniaeuropea can rapidly absorb the contaminants, in rather high concentrations which is related to the duration of exposure and the amount of contaminants. Phenanthrene was selected as a quantitative indicator but more studies should be done in order to find out the efficacy of this plant for bioremediation of other contaminants. In addition, our study showed that Salicornia can be used as a phytoremediator plant to absorb the crude oil contamination specially PAHs in contaminated coast lines. Due to survival advantage of this plant in the contaminated areas, it is suitable to cultivate the endemic variety of this plant in the contaminated coast lines.