1. Background
Multiple Sclerosis (MS) is an autoimmune inflammatory demyelinating disease of human central nervous system. Experimental study on different aspects of MS in animal models dates back to the first half of the 20th century (1). The first animal model was adult monkeys that were immunized with rabbit brain extracts (2). At that time, the clinical statement was called acute disseminated encephalomyelitis, a term that was later changed to experimental allergic or autoimmune encephalomyelitis (EAE) (3, 4). Different procedures, including active or passive immunization are used to induce EAE. Currently the routine procedure is to immunize with myelin proteins like myelin basic protein (MBP), and adjuvants that can be passively transferred to animals by CD4+ T cells (5). Active immunization in the mouse involves immunizing the mouse with spinal cord homogenate or purified myelin peptides. Passive immunization involves adoptively transferring sensitized lymph node cells from immunized animal in naïve mice. Whether passive or active, most EAE immunization protocols result in acute EAE (6). There are many factors contributing in the outcome of modeling, including sex, age, type of animals, anti-myelination proteins, site and method of injection, as well as immunization procedures. To our knowledge, the animals, including rat, mice, and monkey were used for modeling. The most used one is female C57BL/6 mice and the more common procedure is immunization with MOG 35–55 in Complete Freund’s adjuvant (CFA), which induce monophasic or a chronic disease (7). Although same or near to same procedures were used by researchers, during recent years certain modification were done for better results in the modeling of MS induction. To achieve better results, some researchers mainly focused on gender and age of the animals; others believed that the injection procedures may affect the outcome, while some other certain factors may exists, which could affect the outcome of modeling. There are unexpected consequences in modeling that led to undesirable clinical scores; recently the role of environmental factors received more attention. As they can influence experimental outcome, environmental conditions should recognize and monitor during experiment. The present research was carried out to find the possible effects of local transfer and housing (8).
2. Objectives
The present research was performed to find out the possible effects of environmental factors, including animal transfer, handling, housing, and dark-light cycle on EAE modeling scoring.
3. Materials and Methods
3.1. Induction of EAE
We applied mostly the same procedures as other studies stated. Twenty female mice, 5–8 weeks old, (C57BL/6, 19.4 g) were obtained from Pasteur Institute of Iran. The animals were divided randomly into two groups (n = 10). The animals were immunized subcutaneously with 200 μg of MOG 35–55 peptide (Alexis, Switzerland), emulsified in Complete Freund’s adjuvant (CFA), and supplemented with 5 mg/mL of Mycobacterium tuberculosis (Sigma-Aldrich, USA). Then, they received intraperitoneal injections of 400 ng pertussis toxin (Sigma-Aldrich, USA) at the time of immunization, and 48 hours later (9, 10). Following induction, animals of group one were placed in separated room with the least local transfer, and animals of the second group were placed in the room with other experimental animals having normal local transfer and conditions.
3.2. Clinical EAE Score
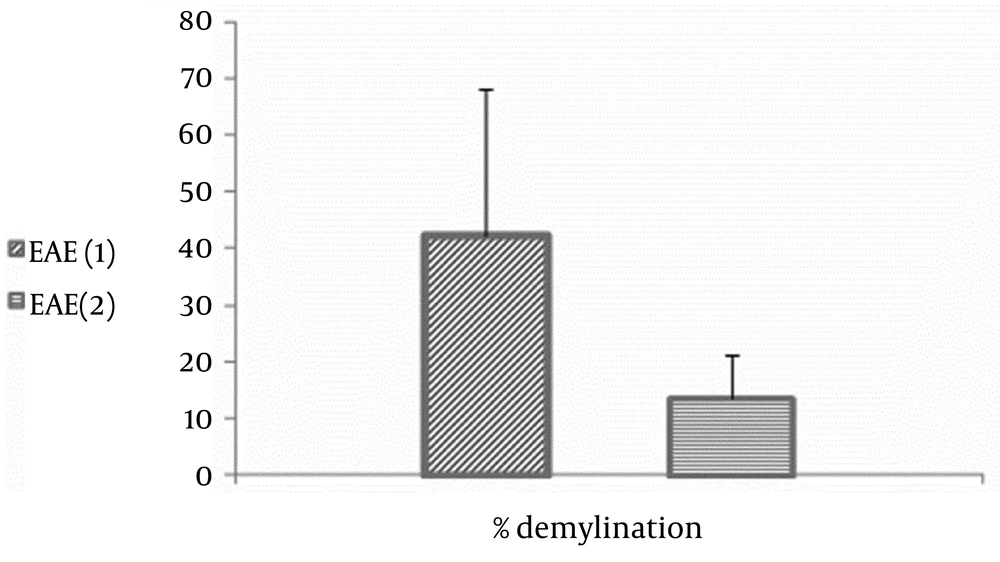
To approve the onset and stage of progression of the disease, the standard scoring system cited elsewhere was used (6). Based on this method, clinical signs of no symptoms, distal weakness or spastic tail and completely limp tail, bilateral partial hind-limb paralysis, complete hind-limb and unilateral partial forelimb paralysis, moribund, and dead were scored 0, 1, 2, 3, 4, and 5, respectively. We confirmed the EAE model by Luxol Fast Blue staining in optic nerve (Figures 1 and 2).
3.3. Tissue Preparation
Perfusion and fixation was done transcardially via left ventricle by aldehyde solutions. Optic nerve was removed and postfixed in the same solution overnight. After that, the optic nerve was exposed to tissue processing and in turn paraffin embedding. By using rotatory microtome, coronal sections of 4 µ thickness, were prepared. To study the myelination, Luxol Fast Blue staining with Nissl contra staining was used. The total surface of demyelinated regions was calculated by Infinity Software (Version 4.4.0).
3.4. Statistical Analysis
By using SPSS V 21(SPSS Inc., Chicago, USA), data were analyzed and presented as mean ± SEM. All mean differences were considered significant if P < 0.05.
4. Results
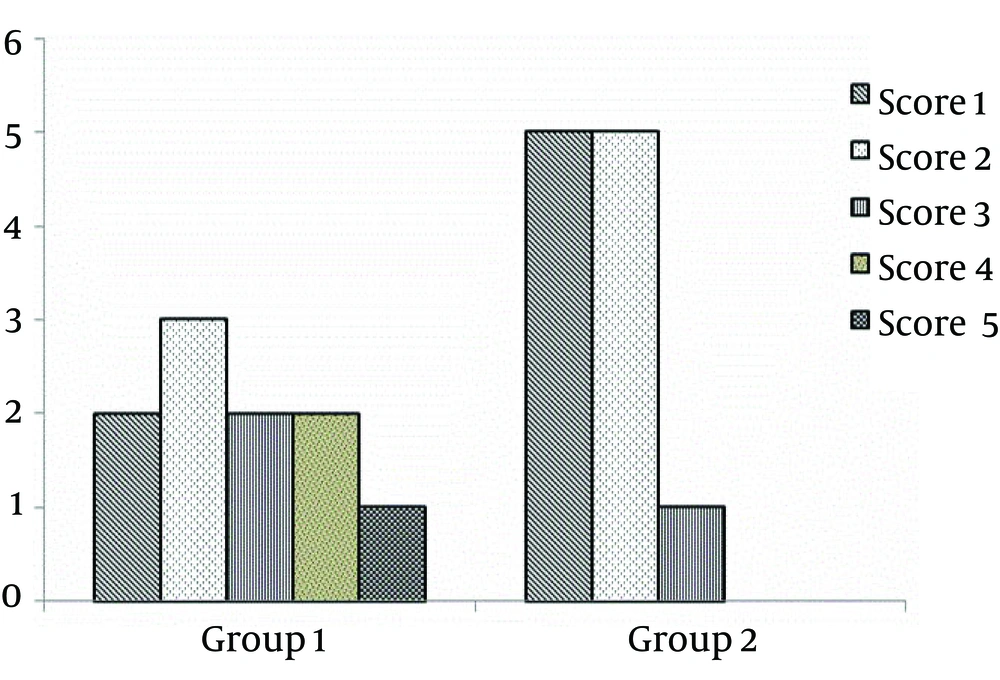
Based on routine clinical scoring for EAE, the animals were observed and scored. The scores of the animals of the first group were arranged as follows: score 1(days 10–12, n = 2), score 2 (days 11–13, n = 3), score 3 (days 12–15, n = 2), score 4 (days 13–17, n = 2), and score 5 (days 15–20, n = 1). And in the second group, they were 5, 5, 1, 1, and 0, respectively (Figure 3). Loss of weight, which was considered as one of the important markers for confirmation of mice model was weighted daily after immunization. The maximum mean score for the EAE in group 1 was significantly lower than the EAE of animals in group 2 (P < 0.05, 17 ± 0.9 vs. 13 ± 0.41, respectively). Our data showed that EAE induction was significantly more successful in the animals of the group one with mean score of more than 2, compared to group two animals (P < 0.05).
5. Discussion
Our results showed that two environmental factors, including method of housing and transfer of the animals influence the outcome of modeling. There are also other reports that emphasize on the role of certain factors that might change the outcome of EAE modeling, including the age and sex of the animals. Other studies like Teuscher et al., was more focused on the method and site of injection (8). Other researchers showed that the season, dark and light cycle, and the time of injection might be important factors that affect the scoring outcome (9). However, Perez-Nievas et al. showed that the EAE modeling is unpredictable and the effect of stress on EAE onset and severity depends on the duration and time of application of the stress protocol and the underlying mechanisms (11). The literature review showed that there are only a few reports concerning the role of animal housing and transfer on EAE outcome. Our data showed the importance of these two factors. We believe that separating housing of the animals after EAE induction may protect the animals from environmental air pollution of the general animal room area that may activate the immune system of the models. It is known that any hyperactivation of immune system might interfere with modeling. Maybe like other autoimmune diseases, exposure to different types of bacteria rendered mice refractory to disease induction (12, 13). Other environmental agents, including trauma, solar radiation exposure, temperature, stress, and toxins, are discussed in terms of their relevance to MS and EAE (14). In addition, the style of animal transfer before and after injection could affect the outcome of EAE modeling via changing the physiological conditions of the animals. We think that a constant place, especially after injection may keep the physiological haemostasis. EAE still is a scientific method to induce MS in rodents, with more attention to certain factors that might influence the result. The mechanisms of the impact of these factors are unknown, but it seems that the role of bacteria should be considered.