1. Background
Since the time of R. S. Cajal, the possibility of new neuron production in the adult brain, neurogenesis, has been adamantly rejected by the scientific community (1). The valuable work of Altman and Das in the 1970s and other neurogenesis evidence provided by Kaplan et al. in the 1990s approved unlike the old idea of not producing and adding new neurons in the adult brain (2-6). Today it is well-accepted that neurogenesis is not limited to prenatal life but that it continues postnatally and into adulthood in certain areas of the hippocampus, including the dentate gyrus (DG) and the subventricular zone (SVZ). Neurogenesis is associated with neuronal plasticity, particularly in the hippocampus; new born neurons that enter into the hippocampal circuitry give rise to functional neurons associated with some types of hippocampal-dependent memory (7). The discovery of neurogenesis gave rise to many new questions. As neurogenesis is the neurobiological basis of memory formation and learning, many scientists studied the factors that may influence neurogenesis. There is growing evidence that shows that various factors such as stressors, immobilization stress, exercise, social isolation, hormones, oxidative stress, metabolic disorders, and the light-dark cycle could affect adult neurogenesis (8-10). Light is the universally constant and powerful stimulus that modulates many types of behavior. Biological rhythms in all vertebrates are modulated by the environmental light-dark cycle (11-14). It has been shown that any changes in the duration of the light-dark cycle or in the light intensity or exposure time could affect the visual system and also brain structures not involved in visual processing (11, 14). Light signals are essential for the maturation of these areas and any disruption may lead to irreversible structural changes (15, 16). It appears that light exposure could affect normal physiology and behavior via the circadian timekeeping system. In fact, social and economic demands have caused humans to become gradually active during the late evening hours, leading to a shift from a primarily diurnal lifestyle to a more nocturnal one. This voluntary decision to stay awake into the late evening hours leads to circadian disruption. These derangements are, in turn, associated with a variety of clinical syndromes. Epidemiological studies show that any alteration in the light-dark cycle, such as those that people experience when working the night shift, is associated with cardiovascular disease, obesity, leukemia, depression, mood disorders, metabolic disease, and cognitive behavior dysfunction (17-20). Recent studies have reported learning and memory impairment following an alteration in light intensity and exposure time. Regarding the role of the hippocampus, it seems that memory and learning deficiencies could be the result of a deficiency in hippocampal neurogenesis (21-23). Fujioka et al. reported that exposure to constant light significantly decreased proliferation in the DG layer of hippocampus. They also showed that exposure to constant light impaired spatial learning task performance (8). Additionally, clinical studies revealed that there is a sex dependency in cognitive performance, learning, and memory disorders (24). The incidence and prevalence of cognitive disorders are higher in women than in men. To our knowledge, most of the research in this field has focused on the effects of light treatment of different exposure times but not on the effects of light deprivation on the neurogenesis in animal models of a single sex. In addition, the extant research has not thoroughly examined sex-dependent cognitive disorders and circadian variation in hippocampal cell proliferation, identification of steroid receptors in hippocampal neurons (25), and the light deprivation sex-dependent effects on neurogenesis.
2. Objectives
The present research sought to discover the effects of sex-dependent, extended, total light deprivation on hippocampal DG layer neurogenesis. Additionally, sexual dimorphism in the DG area was explored.
3. Patients and Methods
Biological model and TLD treatment: Forty male and female adult Wistar rats that received two months of total light deprivation (2mTLD) were used in this study. The animals were sorted into 4 groups (n = 15 for each group) as follows: male control with ordinary light-dark cycle of 10:14 (CM/L/D), female control with same conditions as the male control (CF/L/D), and male and female trial groups kept in total light deprivation for two months, starting one week before delivery and continuing for 7 more weeks (M/TLD and F/TLD). The animals had free access to water and food, and all procedures were approved by the animal care committee for research of the Iran University of Medical Sciences. Nissl staining, BrdU IHC, and MWM were used to evaluate cell density, neurogenesis, and spatial memory, respectively. At the end of month two, seven animals from each group underwent behavioral study using MWM, and the remaining eight animals from each group were immediately perfused and fixed for Nissl staining and BrdU IHC.
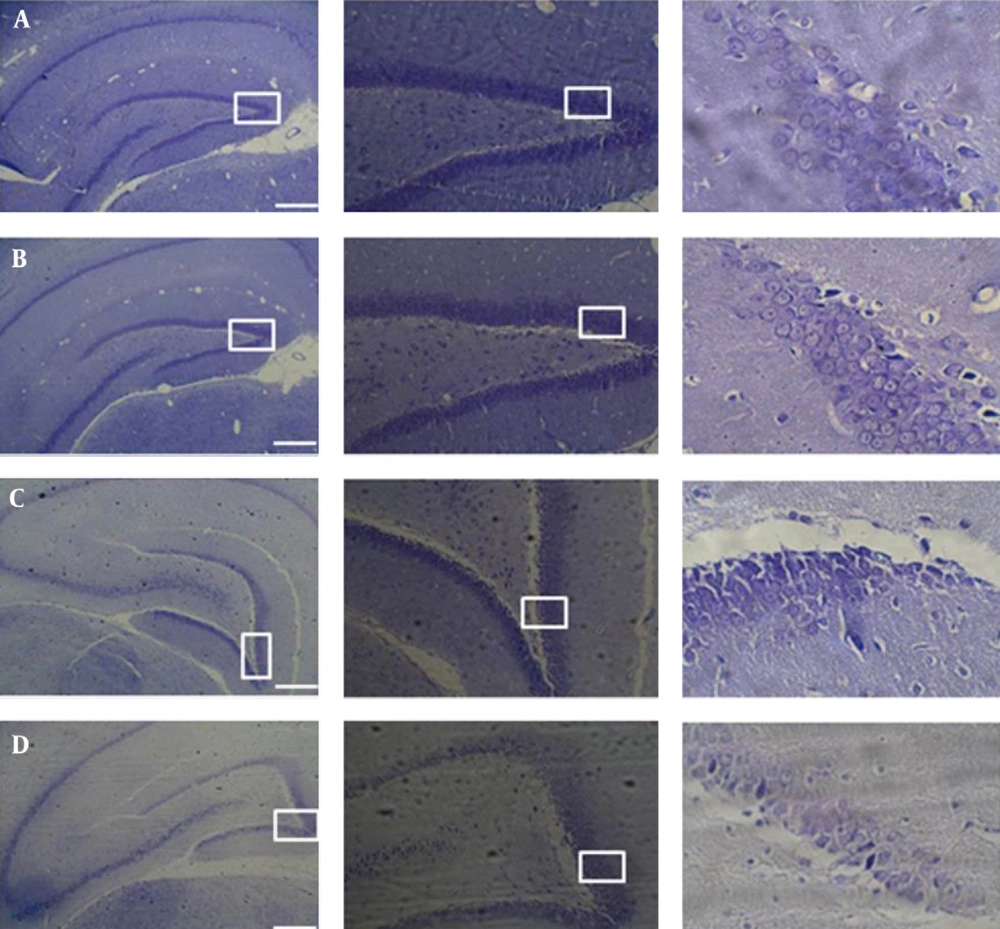
- Nissl staining was used to study neuronal density of the DG layer. To do this, the animals were deeply anesthetized by an intraperitoneal (IP) injection of ketamine 150 mg/kg and xylazine 15 mg/kg. Perfusion and fixation were done transcardially with 300 cc of freshly prepared saline wash, followed by a fixative solution containing 4% paraformaldehyde in a 0.1 M phosphate buffer (pH 7.4), and then followed by a graded sucrose solution in 0.1 phosphate buffer. The brains were removed; coronal sections of 20 µm thickness of the forebrain containing the total length of the hippocampus were prepared using a freezing microtome. According to Paxinos, the DG area of the hippocampus that is located between Bregma -2.12 to -5.30 was studied. The selected mounted sections were stained using the specified Nissl procedures. By using analysis imaging software (Soft Imaging System, Berlin, Germany) at a magnification of 400x, the neurons of DG that showed distinct cytoplasm, clear nuclear outline, and visible nucleolus were counted. The mean for the neuronal number of each group was computed and compared among the various groups.
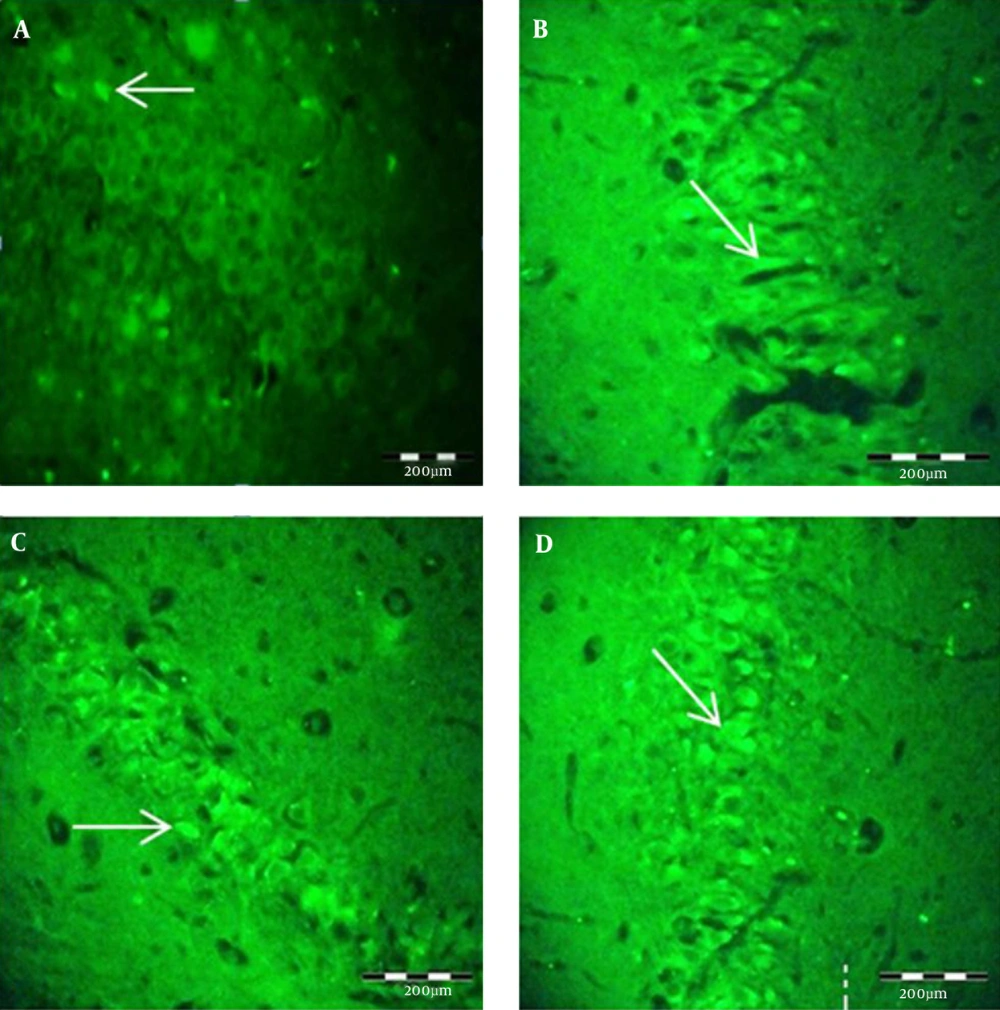
- BrdU IHC was used to study the hippocampal neurogenesis. The procedures of the neurogenesis experiment were performed as previously described (26, 27). A single IP injection (300 mg/kg) of BrdU (Sigma-Aldrich, St. Louis, MO, USA) was administrated to all animals 30 minutes before perfusion. The same perfusion and fixation procedures were done. The brains were removed and processed for paraffin embedding by using rotary microtome serial coronal sections of 4 µm thickness of the total length, and the DG layer was prepared. Paraffin sections were mounted on silane coated slides and then deparaffinized. For DNA denaturation, the sections were incubated in 2x saline sodium citrate (2xSSC) containing 50% formamide for 2 hours at 60°C, rinsed with 2xSSC (10 minutes at room temperature), H2O2, and methanol 1:10 for 20 minutes, incubated in 2N HCl for 30 minutes, and then rinsed with 0.1 M borate buffer (pH 8.5, 10 minutes at room temperature). The sections were rinsed carefully with 0.1M PBS (pH 7.4) 2 times for 10 minutes each and then exposed to a blocking solution containing 0.1M PBS 0.3% Triton X-100/normal goat serum (30 minutes). Incubation with primary rat anti-BrdU was done overnight at 4°C. After two washings with 0.1 M PBS (10 minutes per wash), sections were incubated with secondary conjugated anti-BrdU with fluorescein isothiocyanate (FITC) at room temperature in darkness for 2 hours. After washing with 0.1 M PBS the coverslips were mounted with buffer glycerol. Fluorescent preparations were evaluated by fluorescent microscope, Canon SX30IF (Canon, Inc., Tokyo, Japan). Clear and intense immunoprecipitation within the cells was considered a BrdU immunopositive reaction. By using the Olysia BioReport (Olympus Corporation, Tokyo, Japan) software, BrdU positive cells were counted in the DG area.
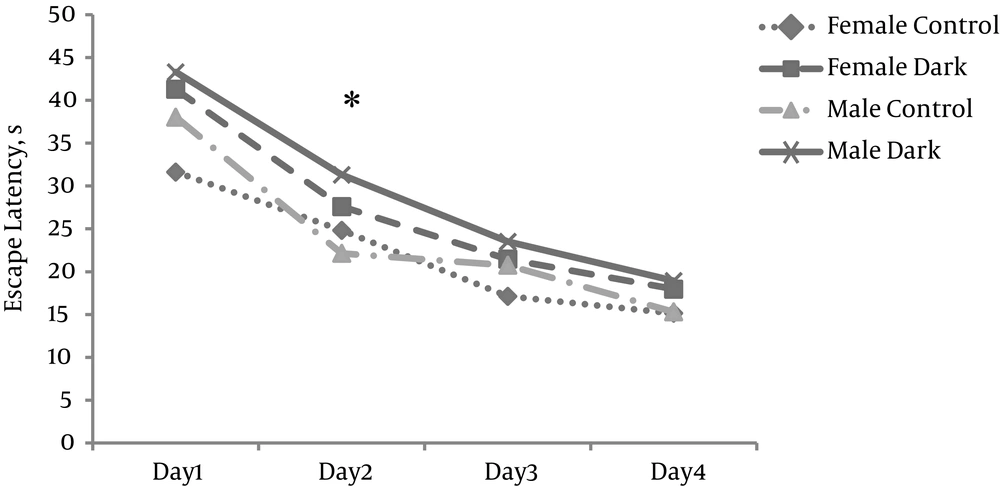
- Morris water maze (MWM) was used to evaluate spatial memory (28). The apparatus consists of a large circular pool, generally being 136 cm in diameter and 60 cm in height and containing water at around 25°C that was filled to a depth of 27 cm to hide the submerged platform (1 cm below the surface of the water). The maze was divided into four quadrants: northeast (NE), northwest (NW). The hidden platform was located in the NW quadrant. A video camera was placed above the center of the pool to capture images of the swimming animal, and this camera was connected to a video or DVD recorder and an online computer system equipped with specialized tracking software. All tasks were done in a dark room between 10:00 a.m. to 3:00 p.m. Each rat was trained for four consecutive trial days. The escape latency (EL/cm) for each rat finding the platform was recorded up to 60 seconds. If a rat could not find the platform, it was manually taken to the platform. In addition to EL, traveled distance (TD/cm) and velocity (V/cm/sec) were also observed and recorded.
3.1. Statistical Analysis
All values are expressed as the mean ± SD. SPSS Statistics 16.0 (Chicago, IL, USA) was used for statistical analysis of the data. Results from Nissl staining and BrdU were analyzed by paired sample t-test, and data from the MWM were examined by a repeated measures analysis of variance (ANOVA), followed by Tukey’s post hoc test. Differences are considered significant when P < 0.05.
4. Results
In addition to the results of TLD on neurogenesis and memory, possible sexual dimorphism in the DG layer of the hippocampus is also presented.
4.1. Results of Nissl Staining
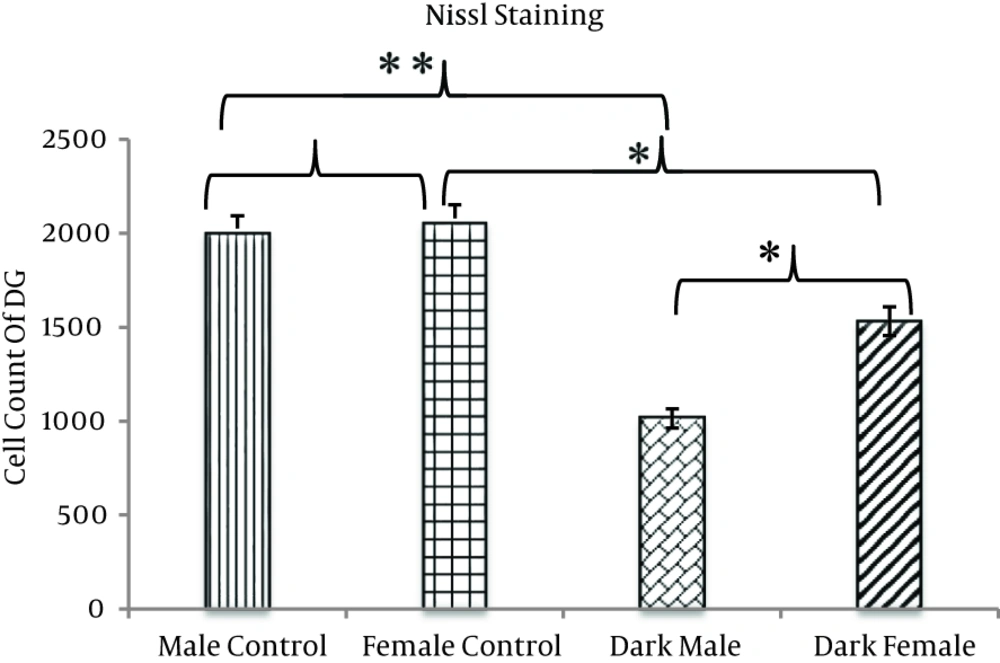
Our findings showed that after 2mTLD, the number of granular neurons of the DG significantly decreased in both sexes compared with animals of the control groups of same sex (P < 0.05). Additionally, there was a significant difference between M/TLD and F/TLD. The number of neurons in the former group was significantly lower than in the latter one. Comparison of male and female controls showed nonsignificant differences (P > 0.05) with a higher number of neurons in the females (Figures 1 and 2).
The number of Nissl positive neurons in the DG is significantly lower in the dark males and females as compared with the control groups. The difference between the two trial groups is significant; the number of Nissl positive neurons in the dark male animals is significantly lower than in the dark female animals. There is a nonsignificant difference between the two control groups, and the number of Nissl positive neurons in the male control animals is lower than in the female control animals (*P < 0.05) (**P < 0.01).
4.2. Results of BrdU IHC Staining
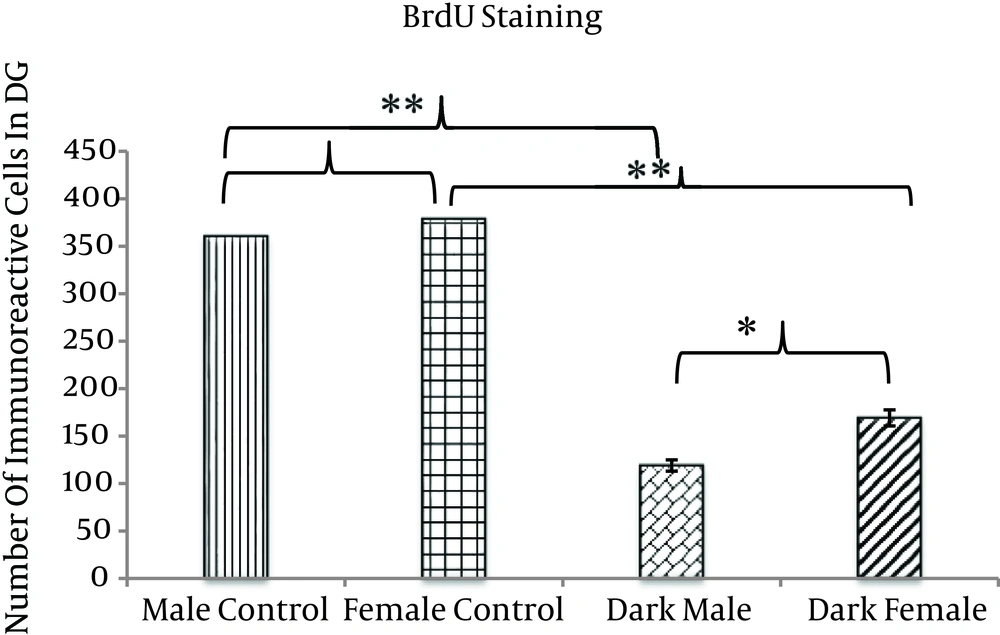
Two months of TLD caused a significant decrease in neurogenesis in the animals of the trial groups, including M/TLD and F/TLD, compared with the control groups. The difference between neurogenesis in the trial groups was also significant; neurogenesis deficiency was more severe in males than in females (P < 0.05). We also found nonsignificant sex-dependent cell proliferation in the DG (P > 0.05). Neurogenesis in this zone was higher in females than in males (Figures 3 and 4).
The number of BrdU positive neurons in the DG is significantly lower in dark males and females as compared with control groups. The difference between the two trial groups is significant, with the number of BrdU positive neurons in dark male animals significantly lower than in dark female animals. There is a nonsignificant difference between the two control groups, with the number of BrdU positive neurons in male control animals nonsignificant and lower than in female control animals (*P < 0.05) (**P < 0.01).
4.3. Results of MWM
To determine whether 2mTLD could affect spatial learning performance, MWM was used. After training, the animals underwent daily trials for four consecutive days. The mean time of EL during trial days (1 - 4) in the trial groups was longer than in the control groups (P < 0.05) (Figure 5). The difference between the two trial groups and the two control groups was not significant (P > 0.05).
5. Discussion
5.1. Discussion of the Sex-Dependent Results of TLD on Neurogenesis and Memory
Our findings showed significant changes in neurogenesis and memory in animals with 2mTLD. We believe that spatial memory deficiency in these animals might be a consequence of decreased neurogenesis. The study also showed that these changes were sex-dependent, so that, in the female animals, the changes were fewer than in the male animals. Together, these findings raise several interesting questions such as how light deprivation could produce these effects and why there are sexual-dependent differences in the results. For the latter, the neuroprotective effects of female sex steroids could be a reasonable answer which will be discussed later, but for the former, light deprivation may act through stress induction or the overproduction of melatonin. In our previous study, we showed the irreversible cellular changes in certain areas of the rat brain, including the visual cortex and dorsal lateral geniculate body following total light deprivation (14). Based on these findings and other similar studies, it can be accepted that exposure to light is necessary for normal development of not only the visual area but also certain other areas not involved in visual processing (29, 30). Regarding the hippocampus, it has been demonstrated that various stressors affect its cellular structure, neural circuits, and functions, including neurogenesis and memory processing. Of these, neurogenesis has received the most attention because of its importance and susceptibility to stressors. Previous studies have shown that hippocampal neurogenesis is affected by a variety of environmental stressors (26, 31-33). It appears that there is a lack of research information about the effects of various light exposure times of light deprivation or constant light exposure on neurogenesis. Fujioka et al. in 2011 reported for the first time the effects of three weeks of constant light on neurogenesis. The study showed that exposure to constant light leads to decreased hippocampal neurogenesis and spatial memory impairment (8). According to that and other previous studies, constant light exposure produced stress response conditions that induce hippocampal neuronal changes and also inhibit cell proliferation in the hippocampus (34-36). Additionally, circadian variation in hippocampal neurogenesis in the granule cell layer (GCL), subgranular zone (SGZ), and the hilus of the DG under cyclic light-dark conditions has been reported recently (21, 23). Thus, it seems logical that any changes in the light-dark cycle, either constant light or light deprivation, might lead to decreased neurogenesis. Presently, we cannot claim that the TLD similar to that reported by Fujioka et al. regarding constant light could act as a stressor or not. However, any alteration in the normal lifestyle of animals and humans could act as stressors to some degree. From this perspective, there are some known mechanism(s) supposedly involved in stress-related responses for neurogenesis and cognitive dysfunction. It has been shown that hippocampal neurons express certain types of steroid receptors such as glucocorticoids, estrogens, and androgens (25). Dranovsky and Hen indicated that psychosocial stress decreases neurogenesis via activation of the hypothalamic-pituitary-adrenal (HPA) axis of the neuroendocrine system and by stimulation of the glucocorticoid receptor (10). In fact, under stressful conditions, neuroendocrine mediators may be involved in the observed effects. Fujioka et al. reported excessive glucocorticoids following stressful conditions that suppress hippocampal neurogenesis (26). How these hormones act is a matter for debate. It has been shown that they not only exert genomic effects (37), but they also act at a nongenomic level via G protein-coupled steroid receptors located in cell membranes (38). In addition to the stress-mediated hypothesis of neurogenesis, other mechanisms have been suggested as being influences upon hippocampal neurogenesis. For example, it is said that different experiences in tactile stimulation or any alteration in exercise and physical activity under varied conditions of the light-dark cycle could produce dramatic effects on neurogenesis (9, 39-41). Because of the important role of light-dark cycle circadian rhythms on physical activity, it appears that light deprivation conditions may affect physical activity (42). Molecular mechanisms such as the brain-derived neurotrophic factor and second messengers are suspected of affecting physical activity on neurogenesis (39-42). Furthermore, we believe that melatonin may also play a significant role. Melatonin, an endogenous signal of darkness, is known as a dark hormone, and it is an important component of the body’s internal timekeeping system. Many roles are suggested for melatonin as it regulates certain physiological behaviors and acts as a powerful antioxidant. Melatonin exerts its effect on physiological actions via the membrane G protein coupled MT1 and MT2 receptors and intracellular proteins. Melatonin receptors found in various parts of the central nervous system (CNS) including the suprachiasmatic nuclei, hippocampus, cerebellar cortex, prefrontal cortex, basal ganglia, substantia nigra, ventral tegmental area, nucleus accumbens, and retinal horizontal, amacrine, and ganglion cells (43). Despite melatonin’s protective and antioxidant effects, it has been reported that its overproduction might have adverse effects. In fact, the length of light exposure time identifies the level of melatonin production or pineal activity. So, short days or partial light deprivation are encoded by a relatively long duration of elevated melatonin secretion; long days or partial constant light are encoded by a relatively short duration of continuous melatonin secretion (44-46). Many studies have shown that exogenous melatonin treatment is able to decrease or block LTP activity in hippocampus CA1 neurons, as well as impair spatial learning and memory (47-50). Although melatonin level was not assayed in the current study, we believe that TLD caused the duration of pineal melatonin excretion that, in turn, affected neurogenesis. Finally, similar to what was reported by Fujioka et al. it is accepted that the learning and memory impairment observed in the present study may be due, at least in part, to the alteration in hippocampal neurogenesis.
We also observed significant sex differences between neurogenesis and memory impairment following 2mTLD, so that neurogenesis and memory impairment were significantly more severe in male animals compared to female animals. In addition to their reproductive role, gonadal hormones have effects on sexual dimorphism, brain development, cognitive behaviors, and memory, etc. (48). Gonadal hormones also exert a wide variety of cellular effects in the non-reproductive context by interacting with several molecular and cellular processes. For instance, gonadal hormones appear to have an influence in the proliferation, development, growth, differentiation, and maturation of neurons (51). The neuroprotective role of female gonadal steroids is one of their most important and well-documented effects. In addition to antiapoptotic effects, they also induce cell proliferation in certain areas of the brain, including the subventricular zone (52, 53). The mechanism of gonadal steroid actions in the hippocampus is not fully understood, but it appears to be receptor-mediated via estrogen receptors (ERa, ERb) and plasma membrane estrogen receptors. The existence of steroid receptors in hippocampal neurons suggests that ovarian gonadal and adrenal hormones are able to modulate hippocampal neurogenesis in adulthood (25, 54). Regarding testicular hormones, it is shown that testosterone treatment or castration presents relatively the same effects as ovarian hormones (55). Sandstrom et al. have demonstrated that the density of dendritic spines in the CA1 region of the hippocampus is positively modulated by testosterone (55). In contrast, castration causes a 50% decline in spine density, an effect that is reversed with the acute administration of either testosterone or dihydrotestosterone (56).
It has also been shown that an adult animal’s castration results in a disturbance in spatial learning retention in MWM tasks (53, 54). Cognitive impairment reported in aging men with a gradual decrease in testosterone and estradiol levels may be due to cellular changes in hippocampal circuits (57). Unlike ovarian steroids, the mechanism of testosterone on the hippocampus is still unknown and needs further investigation; however, common mechanisms for all steroidal hormones may exist.
5.2. Discussion on the Results of Sexual Dimorphism of DG Layer
As a related result in the present research, it was found that there was sexual dimorphism in neurogenesis, cell density, and spatial memory function in the DG layer between control male and female animals. Sexual dimorphism is a well-documented fact in neuroscience literature, and, based on this, the brain is seen as a target for sex steroid hormones that easily pass through the blood-brain barrier and affect reproductive and non-reproductive related areas in such a way that sex-dependent differences could be seen in the structures and functions of certain areas of the brain. Several brain sexual dimorphic structures reported in recent years are far from the scope of this study; however, in our opinion do not exist enough studies about hippocampal sexual dimorphism. Some animal behavioral and human epidemiological studies clearly show sex differences in cognitive tasks and memory that may resemble sex differences at the cellular level of the structure responsible for cognitive processing, i.e., the hippocampus. The hippocampus exhibits important sex-dependent behavioral and physiological functions. Some identifiable sex differences including hippocampal volume and size, neuronal density, and morphology have been reported (58-61). There are also certain sex-dependent hippocampal-associated behaviors such as cognitive tasks, spatial learning, and stress response (62-64). The majority of these reports emphasize total hippocampal sexual dimorphism, and only a few studies exist that evaluate sexual dimorphism in different parts of the hippocampus. Bowers et al. in 2010 reported developmental sex differences in hippocampal neurogenesis in DG, CA1, and CA2 that are mediated by endogenous estradiol (65). Based on their findings, cell proliferation was shown to be higher in newborn males than females (65). In fact, in nearly all reported sexual dimorphic structures of the brain, male dominancy was seen. However, our study showed the opposite finding in which the neurogenesis and cell density in female control animals were higher than in males, although the difference was not significant. We do not know the exact mechanisms or the reasons for such a phenomenon, but it appears that the melatonin interaction with sex steroids and possible disturbances in sexual maturation due to exposure to darkness lead to low sex steroid levels and should be considered for further studies.
The hippocampus is a critical brain region responsible for a variety of cognitive functions such as learning and memory. It is also involved in the physiologic and emotional responses to stress. Any abnormalities in the hippocampus are strongly associated with certain clinical syndromes such as major depressive disorder, schizophrenia, and neurologic disease. We believe that these findings have important clinical implications especially for human literature concerning sex steroids and cognitive functioning, mainly in ageing, changes in the light-dark cycle such as those experienced by night shift workers and also in pathological and physiological decreases in circulating sex steroid levels.