1. Background
Global statistics show that the abuse of methamphetamine is gradually increasing, especially within the age range of 15 to 64 years. The highest consumption rate of this drug is in East and Southeast Asia (1, 2). Methamphetamine is a central nervous system (CNS) stimulant and its abuse is increasing among young and adolescent populations in the reproductive age (3). Many studies are conducted to evaluate the effects of this drug on various adult systems (4). Excessive use of methamphetamine damages different organs, including the brain, lung, heart, and liver (5). In addition to the CNS, the side effects of methamphetamine on the reproductive organs were also observed. The desire and ability to mate in male rats decreased with the administration of methamphetamine and, at high doses, it reduced sperm motility (6). Also, it can induce apoptosis in seminiferous tubules and change the quality of sperm. The percentage of normal sperm motility and morphology significantly decrease in animals treated with methamphetamine (7). Spermatogenic cells are more sensitive than neurons, and as soon as they encounter with lower doses of methamphetamine, the apoptosis is induced (8).
Nudmamud-Thanoi and Thanoi (9), showed that the percentage of sperm reduced in animals treated with methamphetamine. They also showed that methamphetamine induced apoptosis in seminiferous tubules. It was observed that reduction in the concentration of sperm and apoptosis induction both depend on methamphetamine dosage (9).
In another study, Lin et al. (5), showed that methamphetamine decreased body mass and testis mass, and induced epididymitis in mice compared with those of the control group. It is observed that weight-loss in the treated mice is due to the impact of methamphetamine on appetite (5). In addition, testosterone levels decreased in the methamphetamine-treated group (5). In total, the results of the current study showed that daily injection of methamphetamine induced apoptosis in the testicles of the mouse, and also decreased testosterone levels and sperm quality. These had a negative effect on male fertility (5). It is well known that P53 tumor suppressor plays a fundamental role in the onset of apoptosis and causes the activation of genes corresponding to apoptosis such as bax and noxe. Today, different methods are being used to treat addiction with medical, pharmaceutical, and/or psychological approaches. Using antioxidants is one of the methods that are significantly considered by researchers.
Carvacrol is an antioxidant approved by the US Food and Drug Administration. Carvacrol is widely used in traditional medicine, edible additives, the food industry, and pharmaceutical purposes (10). Several properties of carvacrol are known including its anti-inflammatory, antimicrobial, anticancer, anti-tumor, and anti-oxidant properties. Recently, anti-depressant and anti-anxiety properties of carvacrol are identified. Since carvacrol can pass across the blood-brain barrier, it can reach different parts of the nervous system and affect its receptors (11). Recently, Aksu et al. (12), evaluated the effects of carvacrol on side effects created by cisplatin. Cisplatin is one of the drugs used in chemotherapy. The drug has some side effects including liver, kidney, and gonads toxicity, as well as reduced sperm motility and the percentage of live sperm. The results of the current study indicated that carvacrol had a protective mechanism and it reduced apoptosis in sex cells by its antioxidant effects (12). Also, Daggulli et al. (11), evaluated the effects of carvacrol on side effects of methotrexate. They showed that methotrexate caused infertility and reduced the sperm count in males whereas carvacrol reduced apoptosis in seminiferous tubules.
2. Objectives
Considering these properties and antioxidant effects of carvacrol, the current study aimed at investigating the effects of carvacrol on spermatogenic cells and testicular tissue in male rats after treating with methamphetamine.
3. Methods
3.1. Animals
In the current study, 32 male Wistar rats (body weight: 200 - 250 g) were purchased from the Faculty of Veterinary Medicine, University of Tehran, and maintained in the animal room of the Iran University of Medical Sciences under controlled conditions in terms of temperature, light, and moisture with 12:12-hour light/dark cycle. The room temperature was maintained at 22ºC and the room humidity was adjusted to 40% - 50% using a humidifier. The study protocol was approved by the Ethics Committee of Iran University of Medical Sciences, Tehran, Iran.
3.2. Experimental Design
A total of 32 male Wistar rats were randomly divided into four groups: The positive control (PC), negative control (NC), sham (SH), and experimental (EX). Three groups (PC, SH, and EX) received 10 mg/kg methamphetamine only for one day. Rats in the PC group were sacrificed after 48 hours, but SH and EX groups received 18 mg/kg/day sesame oil and carvacrol, respectively for 14 days and then on the day 14 were sacrificed and the NC group had no injections.
3.3. Histological Analysis
Rats were sacrificed and the testes were removed and placed in a Petri dish containing saline. After washing, sections of the bilateral testicular tissue were quickly excised and then fixed in the Bouin solution (13). The samples were dehydrated and embedded in paraffin and 4 μm thick sections were cut using microtome (MIC 509, Euromex, Japan). Then, the sections were placed on glass slides, which were kept at 37ºC for 12 hours. The sections were immersed in xylol to remove the paraffin and then dehydrated in serial alcohol dilutions with descending concentration and deionized water. The sections were then stained with routine hematoxylin and eosin (13) prior to histological analysis.
3.4. Western Blot Analysis
The p53 (1C12) Mouse mAb was purchased from Cell Signaling Company (2524; Boston, MA, USA). The testicular tissue was homogenized and extracted with 300 μL of radioimmunoprecipitation assay (RIPA) buffer (Sigma) on ice, then centrifuged at 1500 g for 20 minutes. The protein was detected by Western blotting with a 90-minute run on 10% gel. Then, it was incubated with mAb for one hour and after washing, 1/6000 secondary antibody was added and treated for 24 hours and then the observed bands were evaluated.
3.5. Statistical Analysis
Data were expressed as mean ± standard deviation (SD). To compare variables between the groups, one-way ANOVA and Tukey post hoc test were used. Data were analyzed with SPSS version 21 and P ≤ 0.05 was a statistically significant value.
4. Results
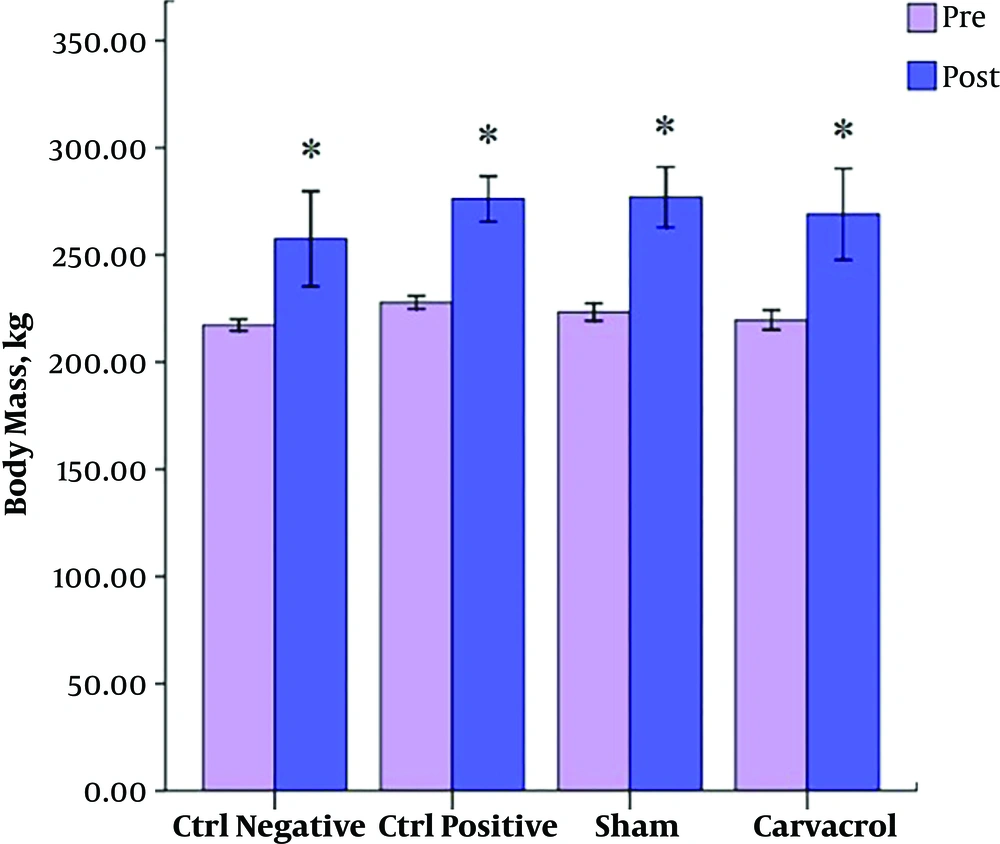
In the current study, there was no significant difference in the body mass of the rats in all groups at baseline, but in post-test, the weight of rats significantly increased in all groups (P < 0.05) (Figure 1).
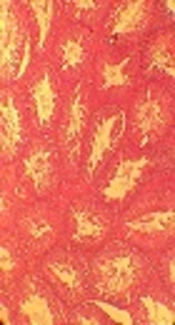
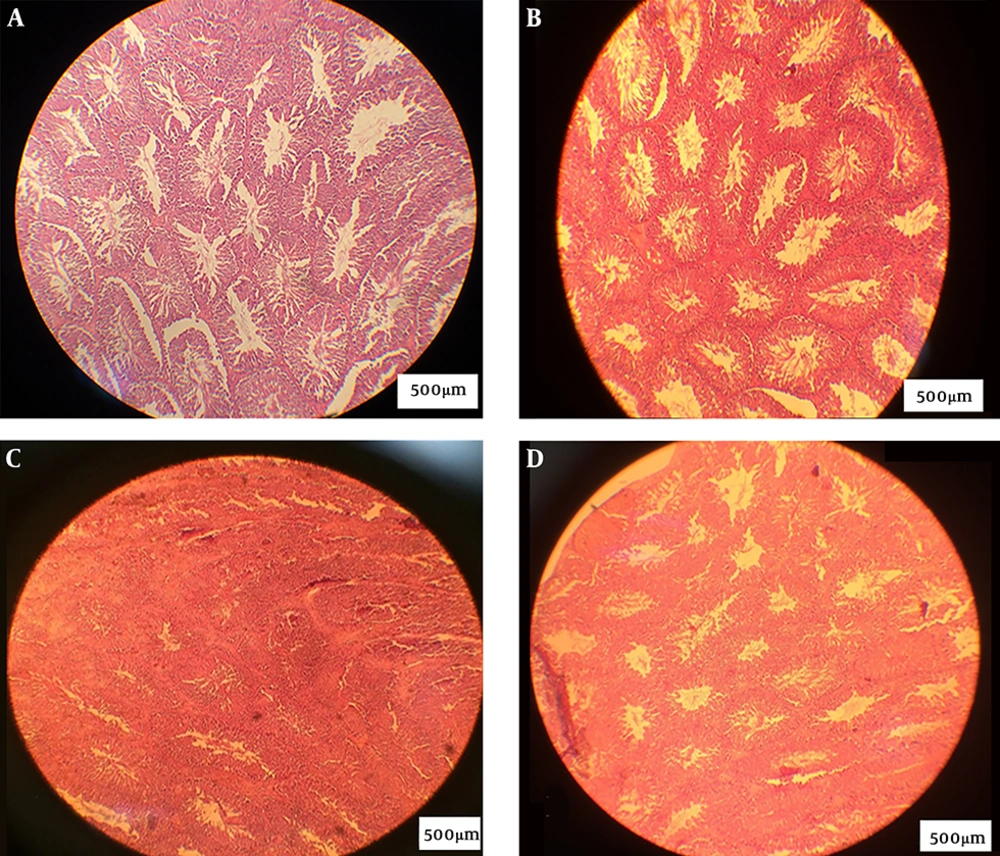
In the NC and SH groups, the lumen was recognized and the tissue was observed intact without distortion. In the PC group, the lumen space was not observed, and testis tissue was also irregular; however, sperm was not observed. In the experimental group, the lumen space was similar to that of the NC group and the lumen space gradually returned to the normal state (Figure 2).
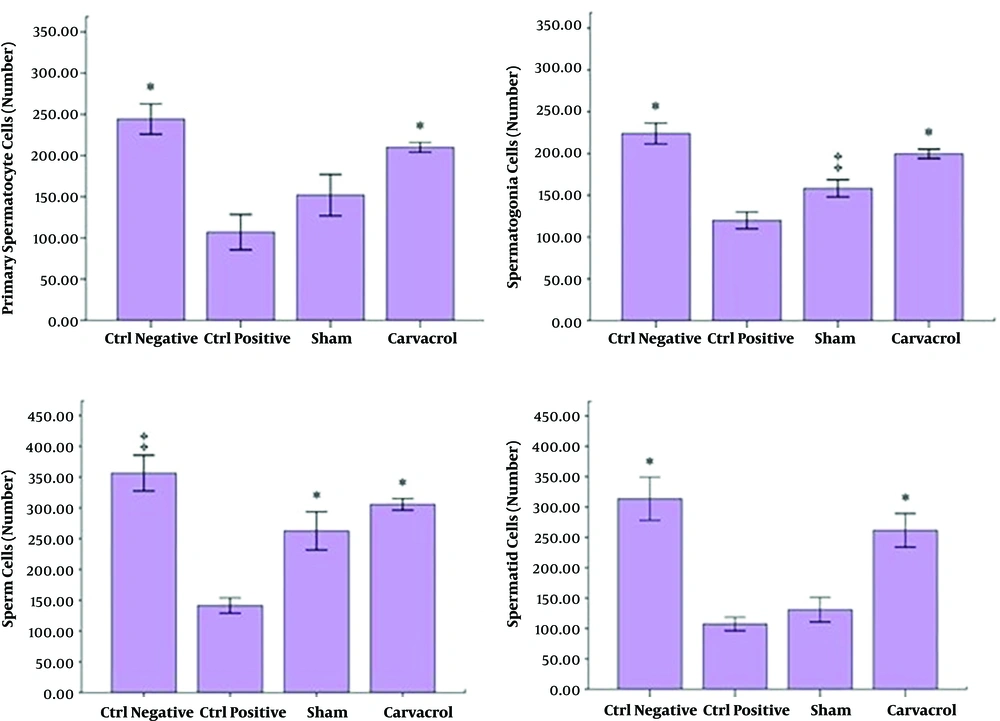
The number of spermatogonial cells in the NC group was significantly higher than those of the PC and SH groups (P < 0.05). The number of spermatogonial cells in the EX group was significantly higher than those of the PC and SH groups (P = 0.001), although there was no significant difference between SH and NC groups (P > 0.05) (Figure 3).
The number of primary spermatocyte cells in the NC group was significantly higher than those of the PC and SH groups (P = 0.001). The number of these cells in the EX group was significantly higher than those of the PC and SH groups (P = 0.001), but there was no significant difference between the EX and the NC groups (P > 0.05) (Figure 3).
The number of spermatid cells in the NC group was higher than those of the PC and SH groups and the number of spermatid cells in the EX group was higher than those of the PC and SH groups (P < 0.001), although there was no significant difference between the EX and the NC groups (P > 0.05) (Figure 3).
The number of sperm cells in the EX group was significantly higher than that of the PC group (P = 0.001), but there were no significant differences between the PC group, and NC and SH groups (P > 0.05).
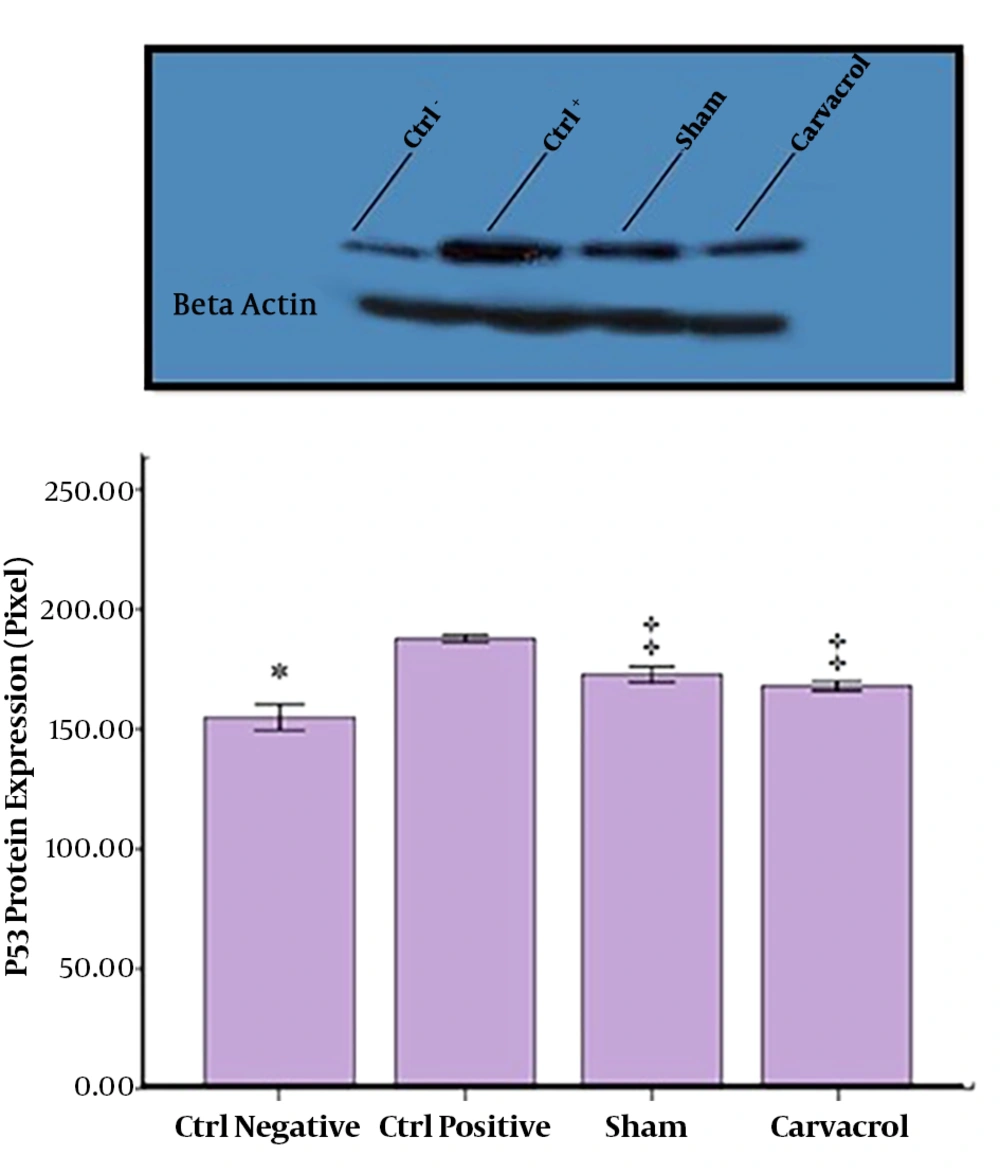
The obtained results showed significant increase in the expression of P53 protein in the PC group compared with the NC (P < 0.05) and EX groups, carvacrol suppressed this expression significantly compared with the PC and SH groups (P = 0.001). There were no significant differences between the NC and EX groups, as well as the NC and SH groups (Figure 4).
5. Discussion
Methamphetamine is a CNS stimulant that its abuse is increasing. It is one of the ingredients of psychoactive pills used in society, especially among the young and adolescents in the reproductive age (1, 2). Today, the use of antioxidants is one of the therapeutic methods considered by researchers (1, 2). Carvacrol is a phenol monoterpene found in many aromatic plants belonging to the family of mints such as oregano and thyme approved by the US Food and Drug Administration (14). The current study aimed at investigating the protective effects of carvacrol on spermatogenic and testicular tissue in rats treated with methamphetamine.
Nudmamud-Thanoi and Thanoi (9), concluded that the percentage of normal sperm in animals treated with methamphetamine significantly reduced compared with those of the untreated animals. The results of the current study showed that methamphetamine treatment significantly reduced the number of sperms in male Wistar rats. Lin et al. (5), observed that receiving 10 mg/kg methamphetamine daily for 15, 60, and 90 days reduced the body mass of the rats compared with those of the untreated controls. Also, they found that the expression of Bcl2 protein significantly increased after 60 and 90 days in the rats treated with methamphetamine.
Unlike previous studies, Heidari-Rarani et al. (15), found that methamphetamine significantly increased testosterone levels and reported no significant decrease in body mass and appetite. The results of the current study did not show a change between the groups, only the body mass of the rats increased after 14 - 18 days of the experiment. It seems that the difference in body mass was due to how to get methamphetamine; in the current study, the rats received only one dose of methamphetamine, but in the study by Lin et al. (5), rats received methamphetamine daily for 15 days.
In a study conducted by Daggulli et al. (11), protective effects of carvacrol against methotrexate-induced toxicity were evaluated in testicles of adult male rats. It was concluded that carvacrol improved spermatogenesis. The results of the current study also showed that carvacrol had a positive and significant effect on spermatogenic cell types (spermatogonia, primary spermatocyte, spermatid, and sperm). Aksu et al. (12), found that carvacrol had a protective mechanism and can improve cisplatin-induced injuries such as reduced sperm motility and sperm disorders. In the current study, the difference in spermatogenic cells between the PC and EX groups represented protective effects of carvacrol. It seems that carvacrol had an antioxidant property that increased the number of cells in the EX group compared with those of the PC group. Taghavi et al. (8), showed that a dose of 10 mg/kg of methamphetamine caused a significant reduction in the number of sperms compared with those of the control group. These results were similar to those of the present study. Yamamoto et al. (6), investigated the apoptotic effects of three doses of 5, 10, and 15 mg/kg methamphetamine on seminiferous tubules of rats. By performing the TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) assay, it was indicated that apoptosis in seminiferous tubules increased at doses higher than 5 mg/kg (8). P53, as a tumor suppressor protein, played a fundamental role in the onset of apoptosis. The application of antioxidants is one of the methods significantly considered by researchers. In the current study, the number of sperms in the PC group was significantly lower than that of the NC group, and according to the Western blotting results it was due to the induction of apoptosis in these cells in the PC group, while after the administration of carvacrol, the expression of P53 protein significantly reduced compared with those of the NC and SH groups.
5.1. Conclusions
Carvacrol can reduce the apoptosis of spermatogenic cells in case of abusing methamphetamine by inhibiting the expression of the P53 protein. It seems that in males, carvacrol can be used to reduce some of the common symptoms of methamphetamine abuse such as infertility.