1. Background
Oxygen is vital for all aerobic reactions. The destructive effects of oxygen are related to the production of free oxygen radicals (1). Bio-energy is provided through the process of aerobic metabolism, but along with this metabolic process, harmful sub-products, including free radicals, are produced. Free radicals have one or more unpaired electrons at their outer electron layer, which is why they are very active and reactive and receive electrons in reaction to other molecules to achieve a stable state (2). Therefore, it is expected that the production of radical species will increase during exercise rather than rest. The main radical species are: (a) reactive oxygen species (ROS) such as superoxide (H2O.O2), hydroxyl radical (OH), hypochlorite (HClO) and single oxygen (O2), (b) reactive nitrogen species (RNS) such as nitrous oxide (NO), nitrite peroxide (OONO), and (c) reactive sulfur species (RS2) (3).
The antioxidant defense system consists of two enzymatic and non-enzymatic sections. The enzymatic system plays a major role in protecting the body against damage from free radicals (4). The body's antioxidant system contains superoxide dismutase and glutathione peroxidase and catalase. The superoxide dismutase enzyme (SOD) is the first line of defense of the body against free radicals of oxygen and converts the superoxide anion into hydrogen peroxide. Other important enzymes of this defense system are ROS, glutathione peroxidase (GPX) and catalase. Catalase converts hydrogen peroxide into water and oxygen, and for this reaction; glutathione, thioredoxin and glutaredoxin are used as electron givers (3). Catalase is an antioxidant enzyme that can neutralize oxidants through the decomposition of free radicals and reducing the risk of radical hydroxyl formation (5).
Malondialdehyde (MDA) is one of the major products of the degradation of unsaturated fatty acids, which is considered as one of the indicators of oxidative damage to lipids, including phospholipid membranes (6). On the contrary, it has been shown that exercise can reduce the risk of damage from oxidative stress and inflammatory signals caused by mechanical disorders and oxidizing agents (7).
On the other hand, today, alongside sporting exercises, the use of medicinal herbs has been increased to prevent and cure many diseases (8). One of the most useful plants in this field is saffron, which has many medicinal properties and therapeutic applications (9). The beneficial effects of saffron and its effects on various tissues of the body such as the central nervous system, gastrointestinal tract, liver, kidney, cardiovascular system, hematopoietic system, etc. have been observed in studies (10). Saffron and its active substances have been shown to have antiarrhythmic and anti-ischemic effects (11). One of the things that can produce oxygen in the tissues of the body is the use of doxorubicin. Doxorubicin, with the brand name of Adriamycin, is one of the strongest anticancer drugs used to treat several types of cancers such as carcinoma, lymphoma, and sarcoma (12).
Although apoptosis is important in the proliferation and removal of pre-cancerous cells in adult mitotic tissues, inappropriate apoptosis is recognized as the underlying mechanism of many diseases. In response to apoptotic stimuli, a variety of internal and external signals regulate the expression of genes that control the onset of apoptosis (4). Therefore, considering the importance of designing training programs for the use of crocin (saffron extract), and to avoid the cost-effectiveness and inadequate studies on the effect of continuous training and use of crocin in long term antioxidant resistance, as well as the contradiction with exercise efficiency and the use of crocin oxidative capacity, the present study investigates 8-week exercise and use of crocin (saffron extract) on the amount of antioxidant dismutase, catalase and malondialdehyde of testicular in non-ovarian mice under apoptosis.
2. Methods
In this study, 56 Wistar male rats (average age of 8 weeks and the average weight of 200 - 220 g) were located at the Research and Laboratory Animals Research Center of Ahvaz Jundishapur University of Medical Sciences. After entering the research environment and familiarity with the two new diets and the activity, the rodents were randomly assigned to 7 groups of 8: (1) healthy control group (saline); (2) doxorubicin group (patient); (3) doxorubicin group + periodontal training; (4) doxorubicin group + crocin dose 10 mg (5) doxorubicin + crocin dose 50 mg 6-doxorubicin + periodic exercise + crocin dose 10 mg of 7-group doxorubicin + periodic exercises + crocin dose of 50 mg. Animals were kept in transparent polycarbonate cages of 15 × 15 × 30 cm length made by Razi Rad Company, 12-12 hours dark circle with ambient temperature 2 ± 22°C and humidity of 50 ± 5%. They were also maintained with proper ventilation.
The groups receiving supplement, exercise, and supplement received oral doses of 10 and 50 mg per kg of body weight for 8 weeks. The control group received only normal saline and the same amount of supplement as gavage. (13).
2.1. Practice Protocol
The main training program was warm up initially for 5 minutes, 40 to 50 percent of the maximum speed on treadmill, then with an intensity of 60 percent of the maximum speed in the first week; 65 percent of the maximum in the second week; 70 percent of the maximum speed from the third week to the next. The rats performed five minutes of cooling at 40 to 50% maximum speed. The total time of the training was 16 minutes in the first week, 24 minutes in the second week, 32 minutes in the third week, 40 minutes in the beginning of the fourth week, and at the end, five minutes of cooling at 40 to 50 per cent maximum speed (14). Fourty-eight hours after the last training session and after 10 - 12 hours of fasting, tissue was taken. Rats were infused with a peritoneal injection of a combination of ketamine (90 mg per kg bodyweight) and xylacin (10 mg per kg body weight), and after abdominal cavity ablation, the testicular tissue was carefully removed and washed off with distilled water and weighed. The weighed tissue was immediately put in the freezer at -70°C for measuring the levels of malondialdehyde, catalase, and dismutase. To avoid night-time effect, the sampling was started from the 8th hour to 11.30 pm.
2.2. Texture Preparation
We reconstituted 50 mg of tissue. A 500 μL buffer was added to the tissue and lined with tissue, then the specimens were placed in the refrigerator for one hour. After an hour, the samples were centrifuged for 15 minutes at a rate of 10000 RPM. The supernatant was transferred to a new micro tube and stored in a freezer at -80°C. The measurement of malondialdehyde in testicular tissue was performed by Okawa et al. (1979) (15). We added 50 μL of standard solution to each micro tube. Fifty μL of the solution 4 was added to each micro tube and mixed. One mL of chromogenic solution was added to each micro tube and we placed the micro tube in water for one hour. The micro tubes were then centrifuged in an ice-cold dish for 10 minutes at 4000 RPM. Two hundred μL of pink solution was transferred to the microplate and the absorbance of the samples was measured at 535 nm and based on the standard curve, the results were expressed in terms of nanomol per gram of tissue. Absorption of catalase (CAT) with a wavelength of 405 nm was carried out at 37°C and the enzyme activity was expressed in unit per gram of tissue protein (16). Dismutase (SOD), based on the kit manual, the specified volumes of the sample and the prepared solutions, were mixed together for a period of from zero to two minutes, then the optical absorption of sample tubes and standard wavelengths of 420 nm was read (17).
2.3. Statistical Analysis
One-way ANOVA was used to examine the variations between groups and Tukey’s post hoc test was used to determine the difference between the groups. In these studies, a meaningful amount at the level of P ≤ 0.05 was considered to be a zero assumption. All statistical calculations were performed using SPSS software version 20 and Excel charts were used.
3. Results
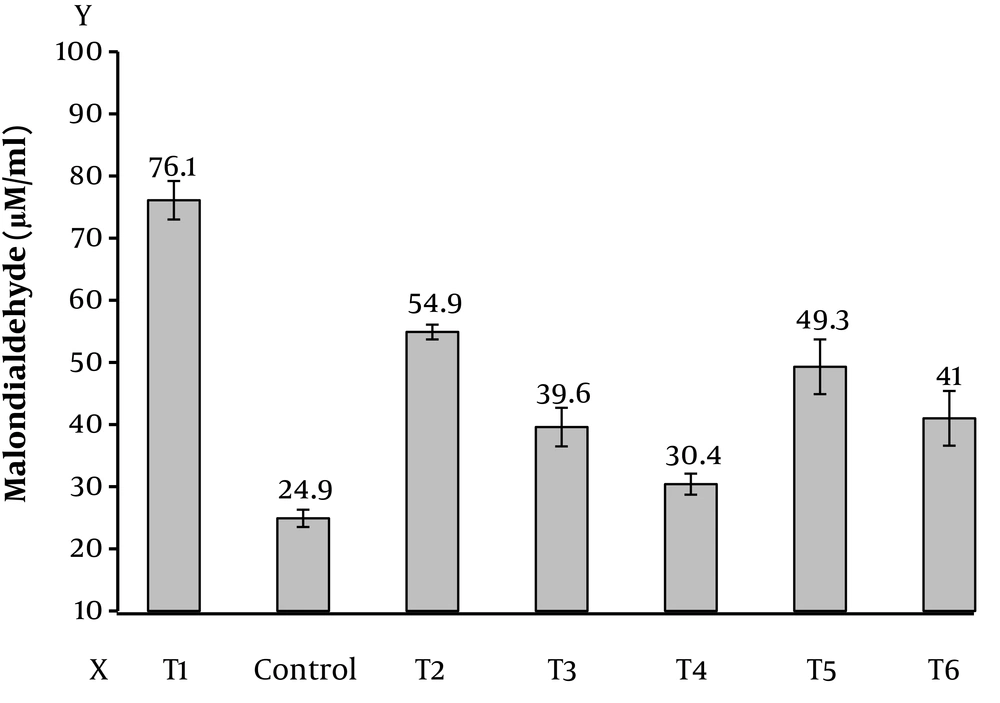
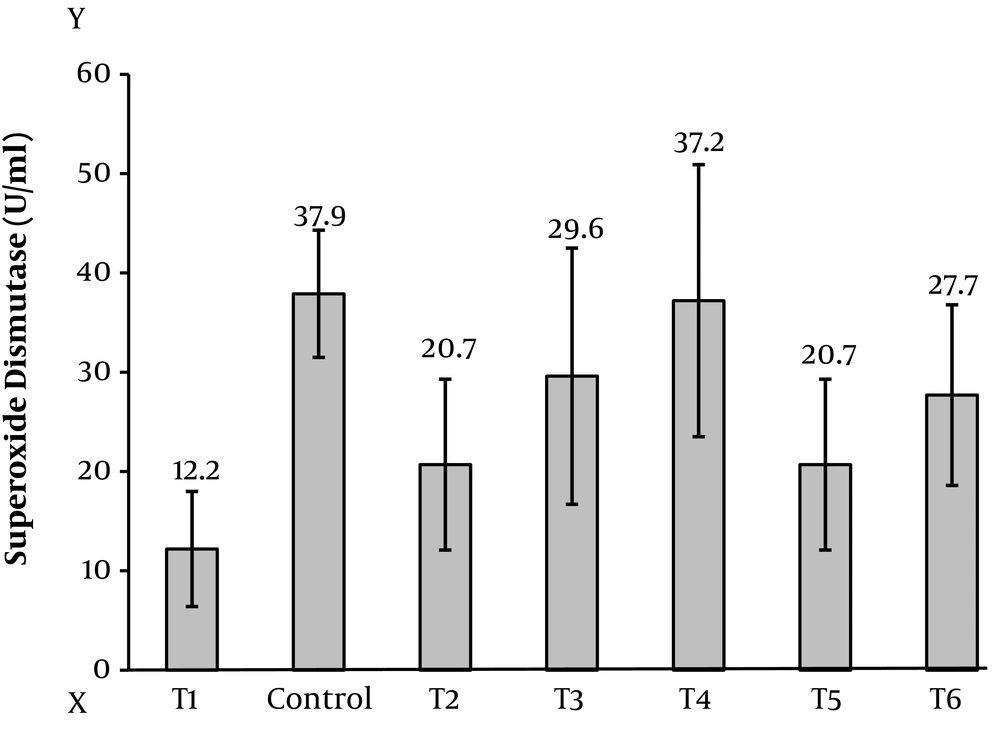
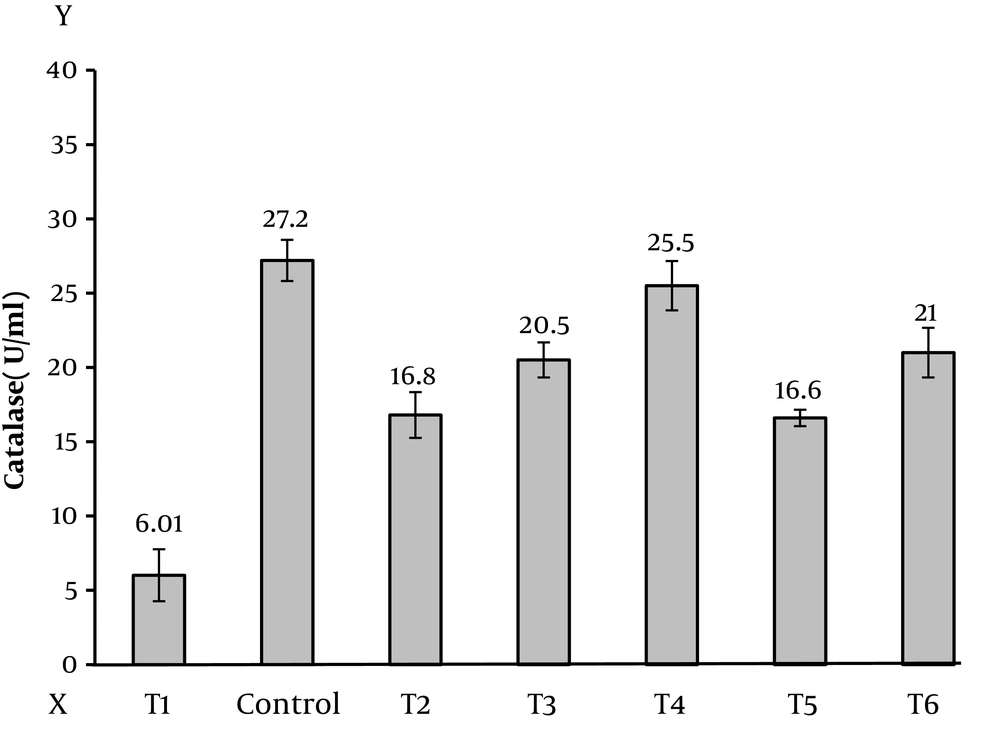
The findings of this study showed that the level of malondialdehyde in testicular tissue is significantly different in all of the control group (Figure 1), this difference is shown in (Table 1). The mean difference at P < 0.05 was significant (Figure 2). The level of superoxide dismutase in testicular tissue according to (Table 1) shows that all the means with control group have a significant difference at P < 0.05, that is, the mean of the other groups is lower than the control group (Figure 3). However, the anti-oxidative index of catalase in testicular tissue (Table 1) shows a significant difference between the groups at the P < 0.05 level, which indicates that the other groups are lower than the control group (Figure 4).
| Variable | Groupa | Test Results | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | T1 | T2 | T3 | T4 | T5 | T6 | F | P Value | |
| Malondialdehyde | 24.9 ± 1.4 | 76.1 ± 3.1 | 54.9 ± 1.2 | 39.6 ± 3.1 | 30.3 ± 1.68 | 49.3 ± 4.4 | 41 ± 4.4 | 248.62 | 0.001 |
| Dismutase | 37.9 ± 4.6 | 12.2 ± 5.8 | 20.7 ± 8.6 | 29.6 ± 12.9 | 37.2 ± 13.7 | 20.7 ± 8.6 | 27.7 ± 9.1 | 7.375 | 0.001 |
| Catalase | 27.2 ± 1.39 | 6.01 ± 1.75 | 16.8 ± 1.54 | 20.5 ± 1.18 | 25.5 ± 1.66 | 16.6 ± 0.55 | 21 ± 1.67 | 187.923 | 0.001 |
Comparison of Mean of Indices of Malondialdehyde, Dismutase, and Catalase in Study Groups
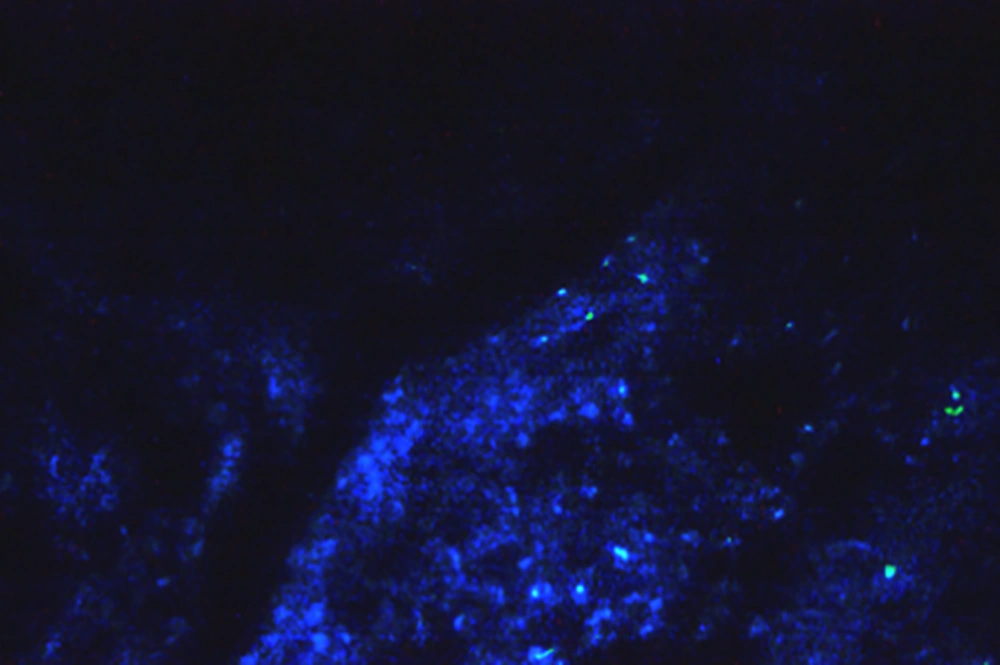
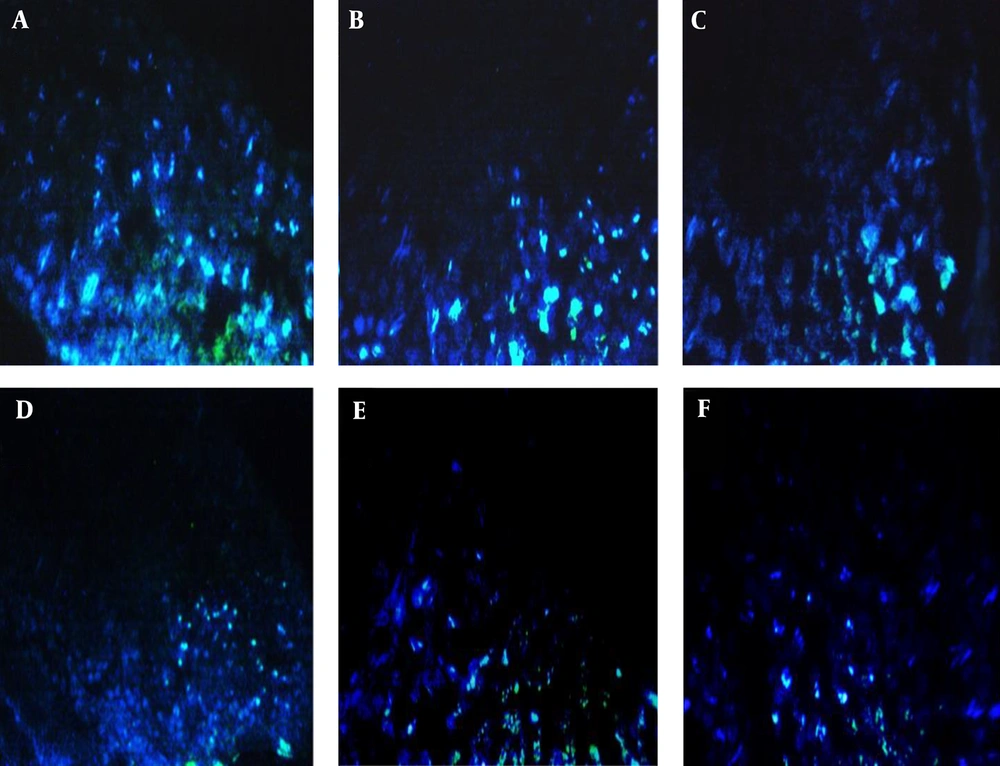
A, Expression of testicular tissue cells in the treatment group (T1) with fluorescence microscopy, with clear blue-green apoptotic points caused by Doxorubicin with a 400-image view. B, Expression of testicular tissue cells in the experimental (T2) group with a fluorescence microscope with low light green and green apoptotic point caused by Doxorubicin, with a 400-image view. C, Expression of testicular tissue cells in the experimental group of (T3) with fluorescence microscopy, with blue dotted areas of degenerated cells recovering from exercise and crocin, with a 400-image view. D, Expression of testicular tissue cells in the experimental group of (T4) with fluorescence microscopy, where the blue points of the cells recovering from the exercises and the crocin, and creating those bright green blue-greenish apoptotic points. The photo view is 200. E, Expression of testicular tissue cells (T5) group with a fluorescence microscope with bright blue spots, cells recovering from crocin, and green spots, degenerated cells, with photo viewer 400. F, Expression of testicular tissue cells in the group (T6) with fluorescence microscopy, which showed intense blue points, cells recovering from crocin, and green points, degenerated cells that were compared to the T5 group are lower due to the higher dose of crocin with a 400-image view.
4. Discussion
The results of histological studies indicated severe testicular tissue damage induced by apoptosis on malondialdehyde oxidative index and antioxidant indices of superoxide dismutase and catalase. Malondialdehyde (MDA) is one of the major products of the degradation of unsaturated fatty acids, which is considered as one of the indicators of oxidative damage to lipids, including phospholipid membranes (6). Increased exercise-induced lipid peroxidation in exercises with intensity of 60, 70 and 80% of maximal heart rate has been shown to show a direct relationship between the intensity of the training cycle and the amount of oxidative stress (18).
In studies carried out by and Popovic, there was a significant decrease in MDA serum concentrations in supplement therapy group, which is consistent with this research (19). While Goldfarb did not see a significant difference in the level of MDA in the training group receiving supplemental vitamin C and control group (20). High-intensity sport activities, through increased production of radical species, lead to oxidative stress and oxidative damage of the lipids and subsequent lipid peroxidation (20). The intracellular accumulation of free radicals leads to peroxidation of membrane lipids, altered cell membrane structure and, as a result, impaired cellular function.
The final product of the peroxidation of lipids is malondialdehyde (MDA) (21). In a study by Bloomer, 30-minute aerobic and anaerobic exercises improved some of the oxidative stress indices and had a different effect on it, so that the magnitude of this effect was different based on the macromolecules under study (22). In the present study, the level of malondialdehyde testicular tissue in all groups was significantly different from the control group (Figure 1). The difference was in the experimental group of doxorubicin and exercise, and the crocin dose 50 (T4) (Figure 5D) had the lowest mean difference of 5.54 and the mean difference with sick group with doxorubicin (T1) (Figure 5A) with the highest mean difference of 51.25 and this mean difference is significant at P < 0.05, which is consistent with Gholami, Babaei and Popovic’s research.
Antioxidants are compounds that inactivate free radicals. They can also protect membranes and organelles from oxidants (23). Therefore, the antioxidant enzymes GPX, SOD and CAT are the first body defense barrier against reactive oxygen species during exercise. Also, further research findings indicate that the activity of these enzymes changes in response to exercise activities in the human body (24). In the present study, the level of superoxide dismutase in testicular tissue, all of which with the control group, was significantly different at the level of P < 0.05. The mean of the other groups was lower than the mean control group. Among the groups, the experimental group received doxorubicin and exercise and crocin 50 (T4) (Figure 5D) with a mean difference of 0.737 and the experimental group received doxorubicin (sick) (T1) (Figure 5A) with the mean difference of 25.72.
Catalase (CAT) is an antioxidant enzyme that can neutralize oxidants by analyzing free radicals and reducing the risk of radical hydroxyl formation (5). While in the study of Gupta, a group that received vitamin C supplement had a significant increase in serum CAT compared to pre-supplementation after an exhaustive (25). Catalase is an enzymatic antioxidant that contains iron as a cofactor that attaches to the active site of the enzyme. This enzyme is widely distributed within the cell. In fact, catalase can be countered by free radical catalyzing H2O2 radical decomposition into water and oxygen and preventing the formation of H2O2. There is a coincidence between CAT and glutathione peroxidase (GPX) function, so that glutathione peroxidase plays a more active role in the removal of this radical in comparison with CAT at low concentrations of H2O2, but when the concentration of H2O2 in the cell increases, it triggerscatalase to act (3).
de Castro et al. stated that activity levels above 60% of the maximum heart rate (anaerobic) reduced the activity of this enzyme (18). It has also been shown that lower oxidation muscle fibers than oxidative wires have lower levels of CAT activity. In this study, there was a significant difference in catalase levels in testicular tissue at the P < 0.05 level, indicating that the other groups were lower than the control group, so that the experimental group of doxorubicin and exercise and crocin 50 (T4) with a mean of 1.71, the lowest and the experimental group treatment (T1) with the mean difference of 21.24, shows the most difference. This research is in line with the research of Joupta, which has been practiced and supplemented, but is not in line with the research of De Castro, Dodd and Sari Sarraf, it is due to anaerobic and speed exercise. Doxorubicin is an anticancer drug, and is used in the chemotherapy of testicular, intestinal, ovary, breast, cervical and prostate cancers (26). Doxorubicin has been shown to inhibit the DNA and DNA-dependent RNA by preventing the splitting of cells by locating two open DNA strands and opening its strands. Doxorubicin also prevents protein production, causing apoptosis and the formation of active oxygen species (27). Previous studies have shown that single dose administration of doxorubicin in mice of 10 mg/kg body weight has toxic effects on ovaries and has reduced the number of primary follicles compared to the control group (28).
Weight loss in doxorubicin-treated patients compared to the control group indicates that the drug affects not only the tissue structure of the ovary, but also other tissues in the body. In this regard, studies have shown that anti-neoplasm drugs are not limited to target cells, and that normal cells are affected. Oxidative stress is one of the other causes of apoptosis. When excess oxidative stress occurs, ROS causes damage to the sperm, because spermatozoa have a low cytoplasm containing antioxidants and are highly susceptible to ROS-induced damages (29). Crocin, crocetin, and Safranal have anti-oxidant properties that can eliminate free radicals and can lead to significant reductions in oxidative damage in ischemic tissues (30). Saffron has an anticancer effect on a wide range of tumors, including leukemia, ovarian and breast carcinoma, large intestine adenocarcinoma, rhabdomyosarcoma, and papilloma (31). Consequently, the results of this study showed that crocin reduced malondialdehyde and increased the activity of antioxidant enzymes of superoxide dismutase and catalase. These effects have been attributed to the ability of crocin to enhance the antioxidant defense system of the body (32). Kemal Duru et al. reported in the year 2000 that it is able to terminate the oxidation reaction of these radicals by removing free radical oxygen intermediates (O2-O and O2) and preventing oxidation of other molecules by oxidizing itself (33). Therefore, the effect of saffron extract in the present study is consistent with these results.
4.1. Conclusions
The use of doxorubicin leads to tissue damage and apoptosis. Following the aerobic exercise and the use of crocin, the cellular defense system is trying to maintain balancewith increasing antioxidant enzymes against the oxidative stress and free radicals. Aerobic exercises with crocin may increase the levels of cell defense and antioxidant enzymes and inhibit the activity of free radicals, and with increasing antioxidant activity, superoxide dismutase and catalase, have beneficial effects on malondialdehyde.