1. Introduction
The human bocavirus (HBoV) was first identified from the nasopharyngeal aspirate specimen in Sweden by Allander in 2005 that includes four subtypes (HBoV1-4) (1, 2). HBoV1 is a major subtype in acute respiratory infections of children and others (HBoV2-4) present in stool specimens (3). HBoV is widespread infection worldwide, which is more prevalent in children younger than 3 years old (4). This virus is currently supposed as a respiratory infection pathogen; however, the gastrointestinal infection caused by HBoV has not yet been confirmed (5, 6). Although several reports have revealed the presence of this virus in fecal samples in acute diarrhea of children, it has not yet confirmed as a gastrointestinal pathogen (4, 6). Because of limitations in HBoV culture and animal examinations, to prove HBoV as a pathogen, clinical studies should be done in hospitals (4-6).
2. Case Presentation
The present study was performed in compliance with the Helsinki Declaration (ethical principles for medical research involving human subjects) and approved by the research board of the Ethics Committee of the Capital Institute of Pediatrics, Kashan University of Medical Sciences, Iran.
The patient’s data were anonymously reported. A 2-month-old boy with acute gastroenteritis admitted to the Shahid Beheshti Hospital of Kashan, Iran. Following history-taking and the clinical examination of the patient, vomiting, alternating fever, and yellow-watery feces from 2 days ago were noted. The infant’s parents reported 10 - 15 watery stools for the last 48 h, and he was severely quiet and pale. Nursing care was provided for the infant at home every day, and he was nourished with powdered infant formula only. The clinical examinations of the cases are listed in Table 1.
| Clinical Examinations | Results |
|---|---|
| Fever, °C | 39.7 |
| Blood pressure, systolic/diastolic | 85/60 |
| Dehydration status | Dry lips and dry buccal mucosa, drowsiness, dullness, and urine output was also decreased. |
| Respiratory rate, breaths/min | between 40 and 80 |
| Oxygen saturation by pulse oximetry, % | 85 |
| Weight, g | 3650 |
The following results were obtained from laboratory tests: hemoglobin: 11.6 g/dL, leukocytosis: (white-cell count, 19800/mm3, lymphocyte, 83%), platelets: 496000/mm3, C-reactive protein was positive, erythrocyte sedimentation rate: was 59 mm/h, sodium concentration: 136 mEq/L, the fecal culture was negative for Salmonella spp. and Shigella spp. The yellow watery stool analysis showed high levels of white blood cells, with no red blood cells, no cysts and trophozoites of Entamoeba histolytica and Giardia lamblia, and no eggs of worms. The stool specimen was negative for rotavirus, evidenced by the enzyme-linked immunosorbent assay.
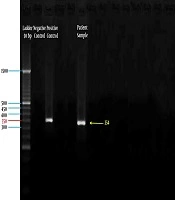
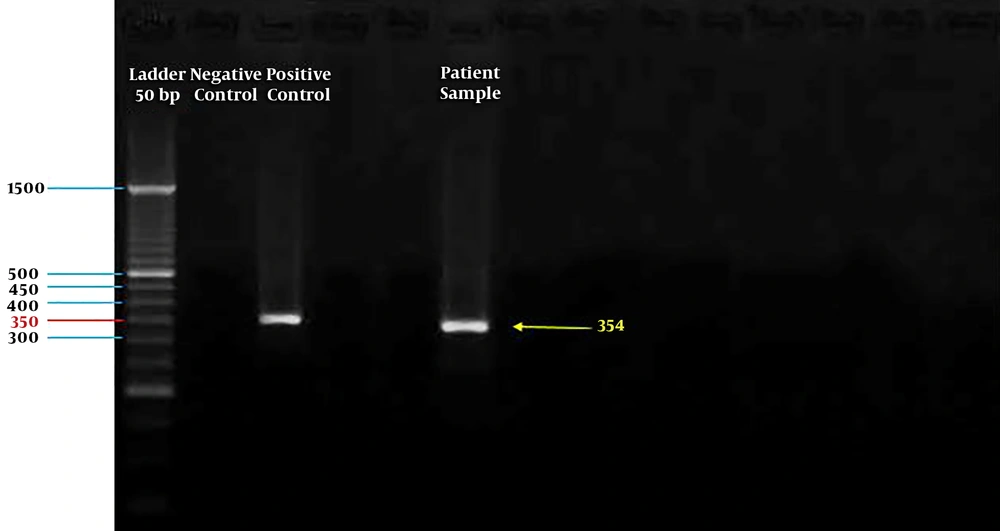
However, the sample was positive for HBoV using polymerase chain reaction assay targeting the NP-1 gene (a 354-bp fragment of the NP-1 gene) (7) and was confirmed by the NP-1 plasmid cloned as a positive control from the Tehran University of Medical Sciences (Figure 1).
Accordingly, the patient was subjected to intravenous fluid therapy, using a solution containing dextrose 5% plus electrolytes. Replacing lost fluid and electrolytes was done with an oral rehydration solution (ORS). To prevent further bacterial infections, antibiotic treatment was considered at the hospital using ceftriaxone 100 mg/kg/day for three days. The patient was discharged from the hospital four days later.
3. Discussion
Acute gastroenteritis in infants is commonly caused by bacterial agents, like Salmonella, Shigella, and Campylobacter, viral agents, such as rotavirus and norovirus, and parasites, like Giardia lamblia, Entamoeba histolytica, etc. (8). HBoV can cause an acute respiratory infection; however, its pathogenic role in acute gastroenteritis has not yet confirmed (4). Although several studies have revealed a high prevalence of HBoV in acute gastroenteritis in children, most of them were co-infection with other viral agents, like rotavirus and norovirus, and adenovirus (4-6). No report has found indicating the uncovered characteristics of the gastrointestinal infection caused by HBoV (9-12). The most common clinical symptoms of the disease in HBoV-positive patients are diarrhea, fever, dehydration, vomiting, and abdominal pain (5, 6).
Regarding acute gastrointestinal infection by HBoV, a study in Pakistan in 2014 revealed the HBoV prevalence of 13%; however, 98% of the cases were found to be co-infected with rotavirus. Amongst the clinical features, fever and vomiting were common symptoms in 89% and 87% of the children, respectively (9). According to the results of a study in Albania in 2016, HBoV was detected in 9.1% of the cases, and all HBoV-positive patients were co-infected with other enteric viruses (98%) (10). In these reports, no data was found on the infection only caused by HBoV and its properties and clinical symptoms.
In a study on children aged 12 months in Western India (2017), 5.3% of the samples were positive for HBoV, and co-infection with rotavirus was observed in 21% of the cases (11). In another report from North India (2016), the prevalence of HBoV was 3% in cases with a median age of 8 months. All positive samples had gastrointestinal symptoms, such as diarrhea (100%), dehydration (86%), vomiting (70%), fever (62%), and severe abdominal pain (28%) (12). In these reports, HBoV was considered as a gastrointestinal pathogen, which was addressed in a separate part of the report.
Similarly, In Iran, Tehran, Mohammadi et al. (13) identified HBoV in 14.4% of the patients with acute gastroenteritis infection. The main clinical symptoms in HBoV-positive patients were diarrhea (83.3%), abdominal pain (81.9%), and vomiting (83.3%).
De et al. (14) in 2017 assessed the association between the risk of acute gastroenteritis and HBoV infection in children. Of the 36 included, the overall prevalence of HBoV in cases with acute gastroenteritis was 6.90%. In the present report, we declared that HBoV could be a gastrointestinal pathogen in children.
In the above-mentioned reports, no distinct information was found to confirm the pathogenic role of HBoV in acute gastroenteritis in children and infants, and all were epidemiological reports. To clarify the role of HBoV as a diarrheal pathogen, more clinical studies focusing on single HBoV infection should be conducted.