1. Background
Alzheimer’s disease (AD) is a degenerative and progressive brain disorder that slowly eliminates memory skills. It is expected that this disease would affect 81 million people in the world in 2040 (1-3). Alzheimer’s disease is explained by the presence of amyloid-beta (Aβ) plaques and neurofibrillary tangles in the brain. The Aβ plaques are extracellular neurotoxic accumulations. Neurofibrillary tangles are intracellular masses of hyperphosphorylated tau proteins, which are cytoskeletal molecules. The Aβ plaques and neurofibrillary tangles can lead to cognitive decline and memory loss (4, 5). There is no adequate treatment for preventing AD. However, stem cell therapy has presented hopeful results in improving AD (6). Various stem cells like mesenchymal stem cells (MSCs) have been used for the treatment of neurodegenerative diseases (7). It is proven that the use of MSCs is free of ethical complications and can possibly be a kind of treatment for AD (8).
The latest studies proposed that the transplantation of MSCs could excite neurogenesis in the brain, secrete growth factors, and increase the endogenous neural stem cells in the dentate gyrus. In addition, the transplantation of these cells could raise the proliferation of recently generated cells in the subventricular zone (9, 10). Another investigation showed that the expression of brain-derived neurotrophic factors was intensely greater in the cell-transplanted animals than in the control group (11).
Mesenchymal stem cells can be derived from bone marrow, umbilical cord blood, and adipose tissue. Human adipose-derived stem cells (hADSCs) are readily and easily accessible, and are less invasive than other stem cells (12). The hADSCs could improve neurogenesis by secreting some neuronal factors like NGF, BDNF, and GDNF (13, 14). The hADSCs are also capable of repairing traumatic neurological lesions (15). Katsuda et al. found that human-derived stem-based cells exuded secretions such as the neprilysin enzyme, which had a significant function in memory improvement by reducing Aβ plaques and inflammatory factors (16).
2. Objectives
In the present study, we aimed to evaluate histological and behavioral alterations following intravenous administration of hADSCs in the AD rat model.
3. Methods
3.1. Chemicals
We prepared Aβ (1 - 42), DMEM, and DiI stain from Sigma (St. Louis, MO, USA). Ketamine (Alfasan, Holland) and Xylazine (Pantex Holland B.V.) were used for anesthesia induction before surgery. Other chemicals in this study were supplied from commercial sources.
3.2. Animals
In this study, we used 32 male Wistar rats weighing 280 - 320 g. Rats were prepared from the Animal House of Iran University of Medical Sciences. The rats were kept under standard conditions with a 12-h light/dark cycle and 18°C to 24°C temperature and they had access to enough water and food throughout the experiment. All experiments were done following the standard guidelines for the use and care of laboratory animals approved by the Ethics Committee of Iran University of Medical Sciences (IR.IUMS.REC1395.9311314007).
3.3. Experimental Design
Rats were randomly selected and divided into four groups of eight rats as follows:
(A) Control group that did not carry any surgery, syringe, or injection; (B) sham group for which, the Hamilton syringe was entered into the hippocampus using stereotaxic surgery, but no injections were made; (C) AD rat model (Aβ), which received intra-hippocampal infusion of Aβ, and (D) stem cell-treated group (Aβ + Sc) that received stem cells through intravenous injection three weeks after intra-hippocampal infusion of Aβ.
3.4. Aβ Preparation and Surgery
We dissolved Aβ1-42 in sterile water to reach the final concentration of 2 mmol/L. Rats were anesthetized by Intraperitoneal (IP) injection of ketamine hydrochloride (80 mg/kg) and xylazine (8 mg/kg) and placed in the stereotaxic frame (Stoelting Co., USA). Each animal received 5 µL beta-amyloid injection in the CA1 hippocampus (AP = 3.8, ML = 2.4, DV = 2.9) according to the Paxinos and Watson atlas (17).
3.5. Isolation and Culture of hADSCs
All hADSCs were isolated from human adipose tissues acquired by the liposuction method from the abdominal superficial layer of 25-45-year-old patients referring to Rasoul-e-Akram Hospital in Tehran, Iran. The patients selected in this study were negative for HIV or hepatitis. The sterile samples were kept in a tube including DMEM/F12, FBS 10%, and penicillin/streptomycin (P/S) 5%. Like our previous works (18, 19), we used a protocol described by Dubois et al. for the isolation of stem cells (20). Before extracting, the fat samples were heated to 37°C in a water bath. The adipose tissue was washed in a P/S 1% solution to remove blood vessels and connective tissues. Collagenase 0.1% and BSA 1.0% were added for tissue digestion.
The samples were centrifuged at room temperature for 5 min at 1200 rpm. Formed pellets were resuspended in BSA 1% solution and recentrifuged. Red blood cells were removed By resuspending with RBC lysis buffer and centrifugation. Finally, cell pellets were washed with PBS, centrifuged, and resuspended in DMEM/F12 culture medium containing FBS 10% and P/S 1%. Then they were transferred to a flask for incubation (37°C, CO2 5%, moisture 98%) until the fifth passage.
3.6. Characterization of hADSC
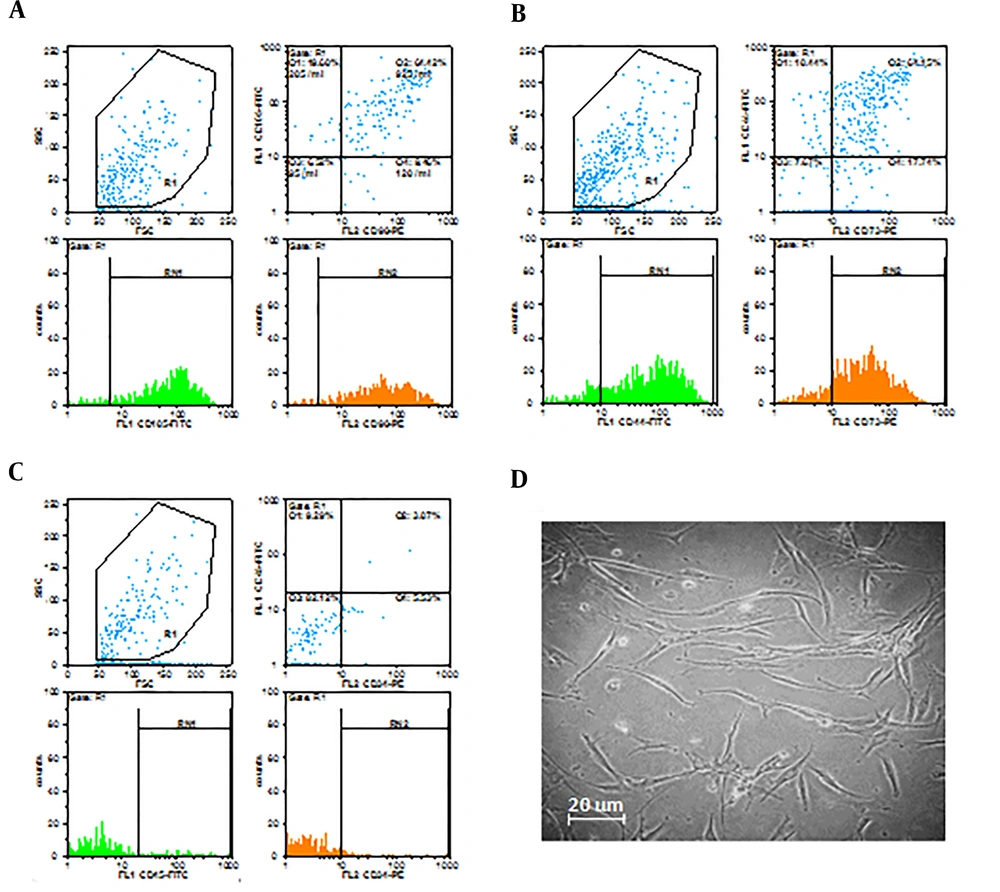
We used flow cytometry for the identification of the hADSCs. The cells were incubated with anti-CD44, anti-CD90, anti-CD105, anti-CD73 (conjugated to FITC), anti-CD34, and anti-CD45 (conjugated to PE) for 30 min. The flow cytometry method demonstrated positive staining for the presence of surface markers of CD44, CD73, CD90, and CD105 and negative staining for the absence of surface markers of CD34 and CD45.
3.7. Labeling of hADSCs
Before injection, in the fifth passage, the cells were labeled with DiI for tracing in the brain. Briefly, 106 cells were suspended to 1 mL phosphate-buffered saline (PBS) and then, 5 µL of DiI solution (50 µg DiI powder dissolved in 50 µL DMSO) was added. The solution was incubated for 5 min (37°C, 5% CO2, humidity 98%). After 20 min of incubation at 8 to 10°C, cells were centrifuged and washed once with PBS.
3.8. Behavioral Tests
Behavioral tests were evaluated by Morris water maze twice: (1) 14 days after Aβ injection for AD rat model confirmation; and (2) Two months after hADSCs injection for evaluating the effect of the stem cells on the AD rat model. The water maze used in this study is explained elsewhere (21, 22).
Swimming speed, distance, and latency to find the platform were recorded by a video camera connected to the Ethovision system (Ethovision XT 7, Noldus Inc., Netherlands). The rats were trained for 5 days. Each day included one block and each block consisted of four trials. In each trial, the animal was placed into the water from one of the four starting points. The animal was allowed to swim for 60 s to find the hidden platform. If the platform was not found by the animal, the rat was directed to the platform by the experimenter. On the sixth day, the platform was removed and the probe test was carried out.
3.9. Nissl Staining
The behavioral tests were done two months after hADSCs injection. Rats were anesthetized with ketamine and xylazine and perfused with 4% paraformaldehyde in 0.1 mol/L phosphate buffer solution (pH = 7.4). The brains were removed and fixed for 24 h in 10% formalin solution. After routine paraffin processing, we prepared coronal sections at 5 µm thicknesses by a microtome. For Nissl staining, the sections were stained with 1% cresyl violet.
3.10. Statistical Analysis
We used one-way ANOVA followed by Tukey’s multiple comparison post hoc tests for analyzing the results. Data are shown as mean ± SEM (standard error of mean). P values of less than 0.05 were regarded as statistically significant.
4. Results
4.1. Stem Cells Characterization
At passage 4, a high percentage of cells expressed specific mesenchymal surface markers CD44 (74.76%), CD73 (81.03 %), CD90 (80.4 %), and CD105 (87.71%). On the other hand, only a small proportion of cells expressed CD34 (8.44%) and CD45 (12.36%) as hematopoietic stem cell surface markers (Figure 1A, 1B, and 1C).
The cell morphology was evaluated with an inverted microscope equipped with a camera, which showed spindle-shaped cells with fibroblast-like appendages (Figure 1D).
4.2. Probe Test for AD Rat Model Confirmation
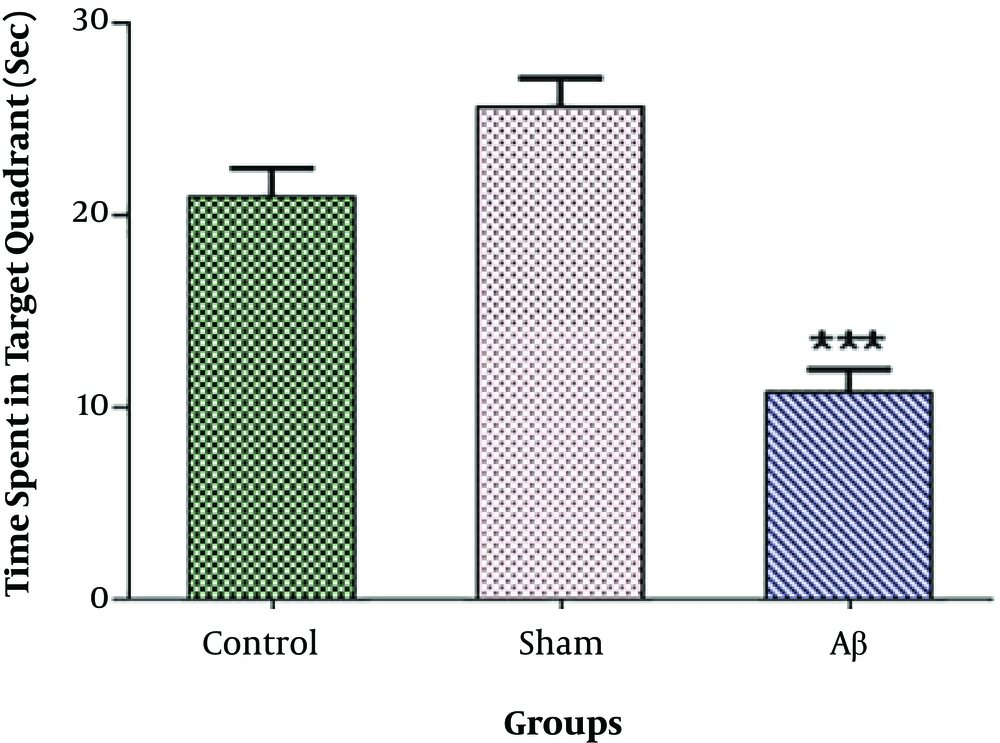
Our data indicated no significant difference in the time animals spent in the target quadrant between the control and sham groups. As shown in Figure 2, this time was significantly lower in the group administered with Aβ into the CA1 region of the hippocampus (***P < 0.001) than in the control group.
The probe test for Alzheimer’s disease rat model confirmation. There was no significant difference in the time animals spent in the target quadrant between the control and sham groups. The administration of amyloid-beta into the CA1 region of the hippocampus significantly decreased this time compared to the control group. Each point shows the mean ± SEM for eight rats. (***P < 0.001 different from the control group).
4.3. Probe Test After hADSCs Administration
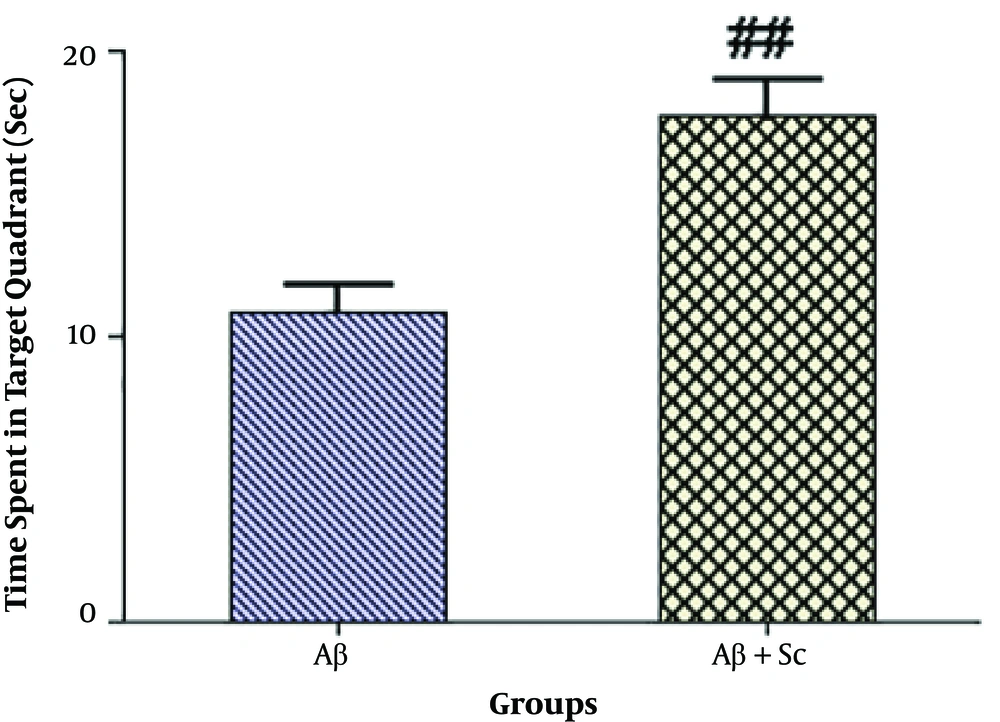
As shown in Figure 3, the intravenous administration of hADSCs significantly increased the time animals spent in the target quadrant in the probe test (##P < 0.01) compared to the AD rat model.
The probe test after hADSCs administration. The human adipose-derived stem cells significantly increased the time animals spent in the target quadrant in the probe test compared to the Alzheimer’s disease rat model. Each point shows the mean ± SEM for eight rats. (##P value < 0.01 different from the amyloid-beta group).
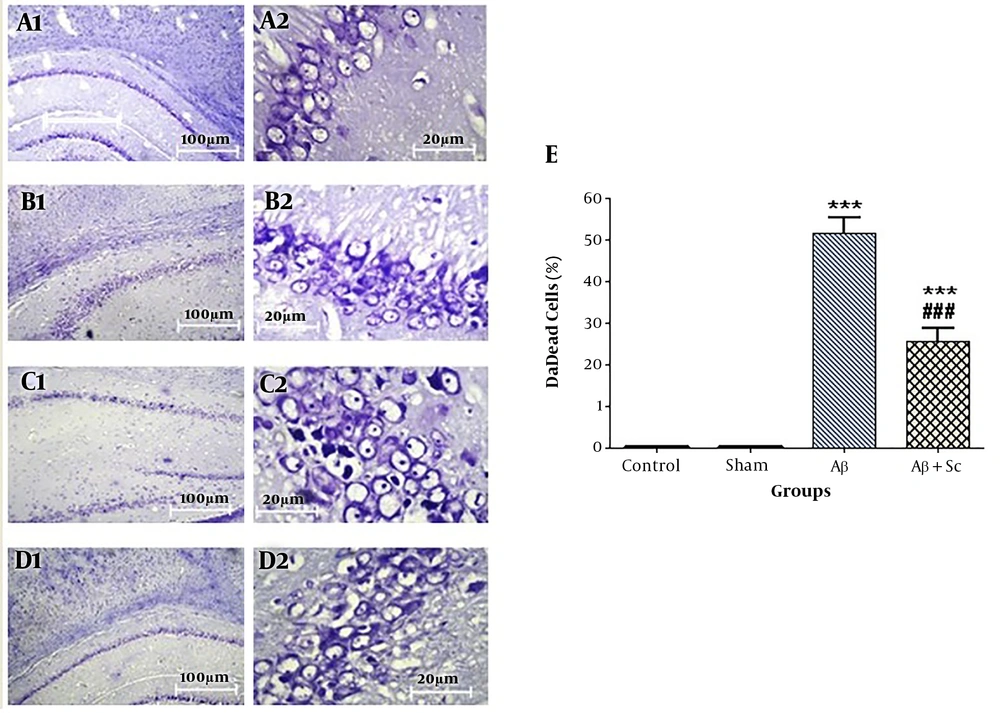
4.4. Nissl Staining
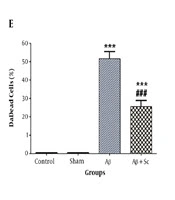
The results of CA1 Nissl staining are shown in different groups with 10× and 40× magnifications in Figure 4A, 4B, 4C, and 4D. Statistical analysis indicated that the counts of dark neurons were the same in the control and sham groups and they were significantly higher in the AD rat model group than in the control group (***P value < 0.001). On the other hand, the number of dark neurons was lower in the hADSCs treatment group than in the AD rat model group (###P value < 0.001) (Figure 4E).
CA1 Nissl staining. A, Control; B, sham; C, AD rat; D, and human adipose-derived stem cells (hADSCs) + amyloid-beta groups (1 shows 10× magnification, 2 shows 40× magnification); E, percentage of dead cells in the study groups. Alzheimer’s disease rat group showed a significant increase in dead cells compared to the control group. Administration of human adipose-derived stem cells significantly decreased dead cells in Alzheimer’s disease rat group. Each point explains mean ± SEM. (***P < 0.001 different from the control group, ###P < 0.001 different from the Alzheimer’s disease rat group).
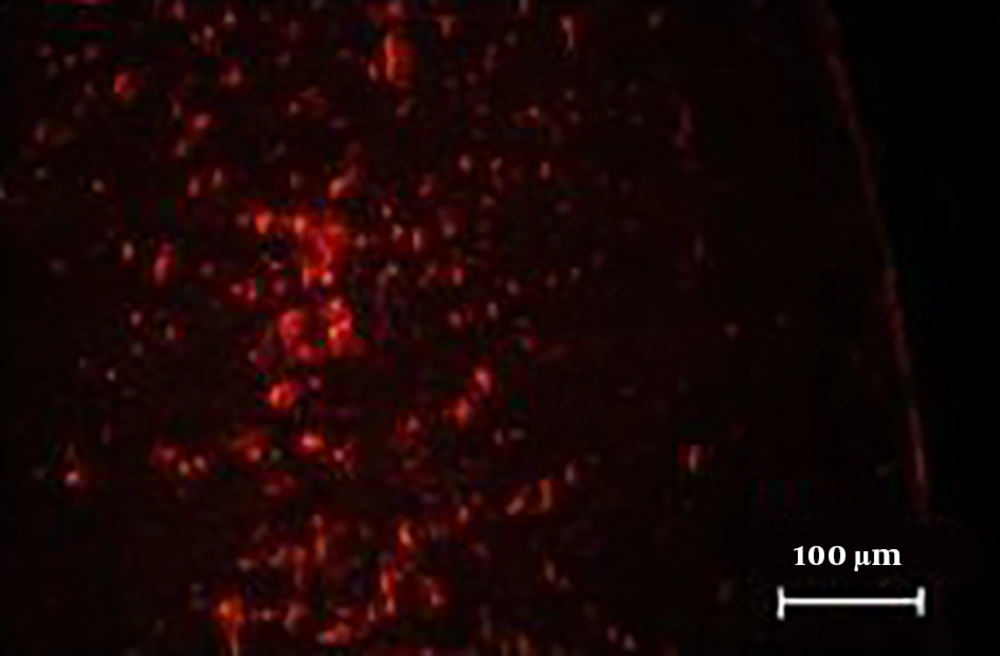
4.5. Homing of DiI-Labeled hADSCs in AD Model
The results showed that hADSCs, which were labeled with DiI, migrated from the site of delivery to the brain and they were detectable with the fluorescent microscope, as shown in Figure 5.
5. Discussion
In this study, through stem cell therapy of the AD rat model, stem cells transferred into the brain, decreased the number of dead cells in the CA1 region of the hippocampus and improved memory. As the blood-brain barrier (BBB) is permeable in AD brains due to its destruction, stem cells can pass through this barrier after intravenous injection (23, 24). Recent results showed that the adipose tissue is a better cell source than other sources of stem cells as it can be collected from various regions of the body with the least side effects by liposuction as a general surgical process (25, 26). The ADSC can be gained in larger measures than other stem cells (27). The ADSCs, which can migrate into damaged brain regions, are more useful for the retrieval of brain injury in contrast to bone marrow stem cells (28). The ADSCs have a significant potential for tissue repair in the broad range of diseases with neuronal damages such as Parkinson’s disease and AD. They have the capacity to differentiate into other cells such as cartilage, muscle, bone, and neuronal cells. There are various mechanisms for improving the effects of stem cells in AD. It had been confirmed that ADSCs could have therapeutic effects by mediating paracrine signaling of angiogenesis, inflammation, cell homing, and cell survival (26, 29, 30). The ADSCs administration presented raised levels of growth factors like basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), nerve growth factor (NGF), and neurotrophic factors such as glial-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor (BDNF) (16, 31, 32). An investigation showed that intravenous-transplanted hADSCs induced the proliferation of endogenous stem cells in the hippocampus of transgenic mice models (24). Earlier reports indicated that glutamate reduced in AD (33), demonstrating the main role of glutamate in mammalian brains, including neuronal plasticity, neurotoxicity, development, learning, and memory (34, 35). On the other hand, in vitro studies revealed that cultured neural stem cells could differentiate into an astrocyte population that expressed glutamate transporters (36). In this study, the effect of hADSCs on improving memory in AD rats could be related to the mentioned mechanisms.
5.1. Conclusions
For the first time, we found that hADSCs could transfer into the brain and improve memory and neuronal damage in the AD rat model. These findings may be related to various mechanisms. Further in-depth studies are still needed to elucidate these interfering mechanisms.