1. Background
Free radicals at physiological concentrations are essential for normal cell function, and their low to moderate increase has a role in regulating cellular signaling pathways (1). Free radicals are found in two types in different cells and tissues of the body. The first group is reactive oxygen species (ROS), the most important of which are superoxide anion, hydrogen peroxide, and hydroxyl. The second group is reactive nitrogen species (RNS), which nitric oxide is one of the most important one (2, 3). In other words, increasing reactive oxygen species (ROS) results in increased oxidative stress and consequently can disrupt the balance of oxidants and antioxidants in the heart tissue (4). As energy production is associated with hydrogen ion production, it increases hydroxyl radicals, thereby increasing pro-oxidant-antioxidant balance (PAB) as an enhancer of oxidative stress and impairing the function of proton vectors such as cytochrome c and ultimately damaging cellular DNA and apoptosis (5). Apoptosis is a programmed cell death involving several physiological and pathological events (6). Various studies have shown that exercise can increase antioxidant capacity and decrease oxidative stress (7). Catalase can convert H2O2 to H2O and O2 in the peroxisome of eukaryotic cells. The glutathione-dependent antioxidant system is composed of decreased glutathione (GSH) and a set of functional enzymes that play a key role in cell defense against reactive free radicals and other ROSs (8). On the other hand, among the ROSs, the hydroxyl radical group causes the peroxidation of fats, among the products of which can be the concentration of cytochrome c and malondialdehyde (MDA) and is considered an indicator of oxidative stress. Dietary factors are believed to play a critical role in the development of various human diseases such as heart and metabolic diseases, atherosclerosis, hyperlipidemia, thrombosis, diabetes, and hypertension (9). In addition to exercise, today, many sport science researchers are interested in the use of natural supplements and medicinal herbs for their low side effects. Herbal drugs are traditionally used in the developed countries. The popularity of the pumpkin plant in a variety of traditional medicine systems has attracted the attention of researchers on a variety of diseases due to its anti-diabetes, anti-hypertension, anti-tumor, antibacterial, anti-cholesterol, and anti-inflammatory and immune system properties. The pumpkin seeds (PS) contain 40% to 50% lipid and 30% to 37% protein (10). Pumpkin seeds are a great source of energy consumed around the world that are a great source of potassium, phosphorus, iron, and beta-carotene (11). Various researches studied the antioxidant effects of endurance training. Researchers believe that exercise can increase antioxidant capacity and reduce oxidative stress (12). For example, regular and prolonged exercise training with 75 to 80% of maximum oxygen consumption had a significant effect on reducing oxidative stress, increasing antioxidants, improving the function of electron transport chain proteins in rats (7). Regular exercise training increases superoxide dismutase and reduces oxidative stress (12, 13).
2. Objectives
Considering the aforementioned findings and lack of a study on protective effects of resistance training (RT) and consumption of PS in H2O2-induced oxidative damage, this research aimed to study the effect of RT and PS on GSH, MDA, PAB, and adenosine triphosphate (ATP) in the heart tissue of rats exposed to H2O2-induced oxidative damage.
3. Methods
In this study, 42 male Wistar rats with a mean weight of 250 ± 50 gr and approximately eight weeks of age were purchased and kept for one week under standard conditions and temperature control (22 ± 2°C), and a 12-hour alternating light/dark cycle. Seven groups of six rats were provided, including (1) control (C); (2) treatment control (TC); (3) RT; (4) 1 g/kg PS (1PS); (5) 2 g/kg PS (2PS); (6) RT + 1PS; and (7) RT + 2PS. Groups 2 to 7 received 1 mg/kg H2O2 peritoneally in eight weeks (manufactured by Sigma Aldrich, USA) (14); groups 4, 5, 6, and 7 received ethanolic extract of PS at given doses by gavage (15); furthermore, groups 3, 6, and 7 performed RT three days a week at 9:00 AM and 2:00 PM (16).
The RT protocol consisted of climbing a ladder of one meter with two centimeters of distance between the two steps and an 85-degree slope that was run in three sets of five reps with one-minute rest between reps and two minutes rest between each set. The initial weight selected was 50% of the rats’ body weight, which was gradually increased by the end of the eight-week period and increased by 100% in the final week (16). Each RT lasted 60 minutes for each rat from 9:00 AM to 2:00 PM.
Pumpkin seeds were powdered by an electric grinder and mixed in 80% pure ethanol in a ratio of 1 to 10 every two hours; After performing these steps, the obtained material was passed through a paper filter with a thickness of 0.2 mm. The rest of the product was placed in the seepage system, and the ethanol was allowed to evaporate. The herbarium number of this herb is available at Islamic Azad University of Central Tehran with code number 1300. It should be noted that the prescribed doses of PS were based on previous studies (15).
At the end of 8 weeks, after 48 hours of the last training session and PS consumption, the rats were anesthetized, and perfusion was done to remove tissues blood following the full anesthesia. To collect the left ventricle of the heart, the rats were sacrificed by cervical dislocation, and the left ventricle tissue was extracted. ATP, GSH (CUSABIO Company, USA, Catalog Number: CUSABIO Company, USA, catalog number: CSB-E12146r), MDA (CSB-E08558r), and PAB (Minneapolis, MN, USA) were measured by ELISA using laboratory kits. One-way ANOVA and Tukey’s post-hoc test (P < 0.05) were used to analyze the data.
4. Results
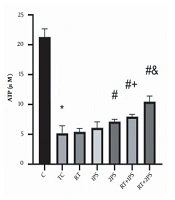
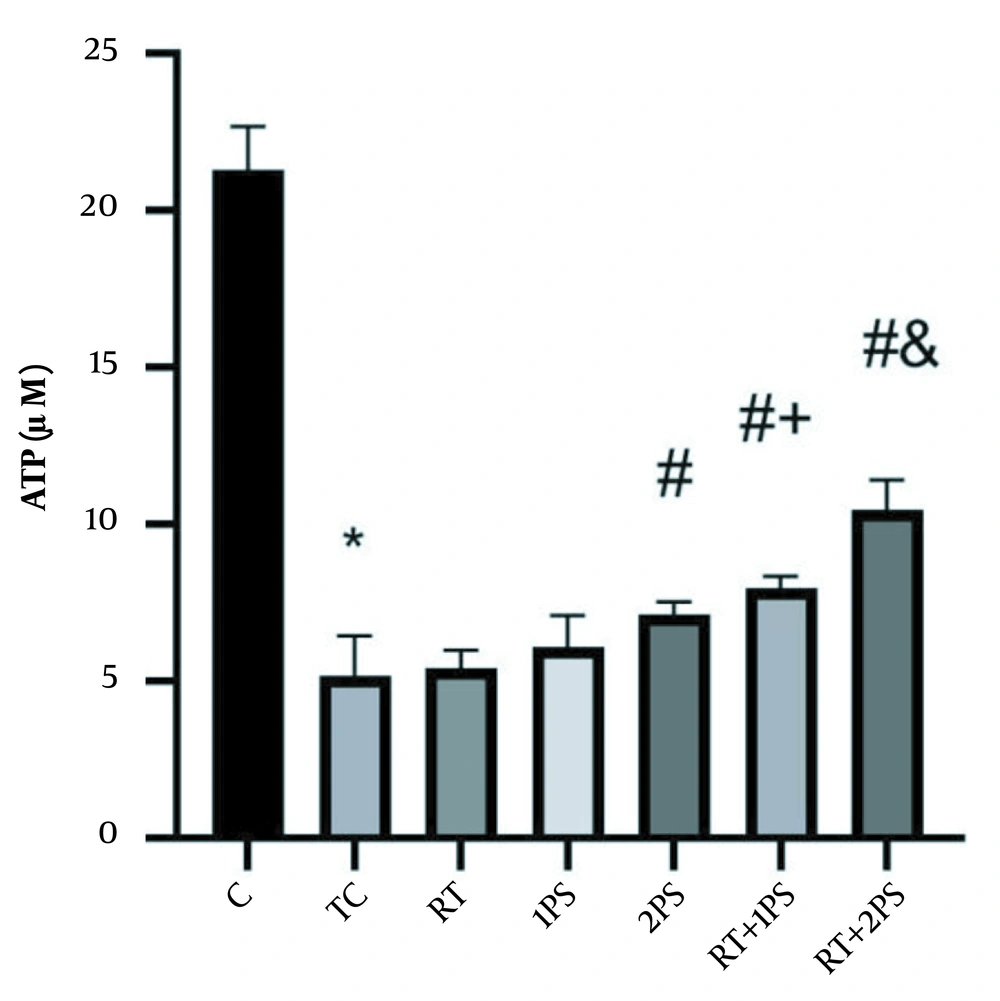
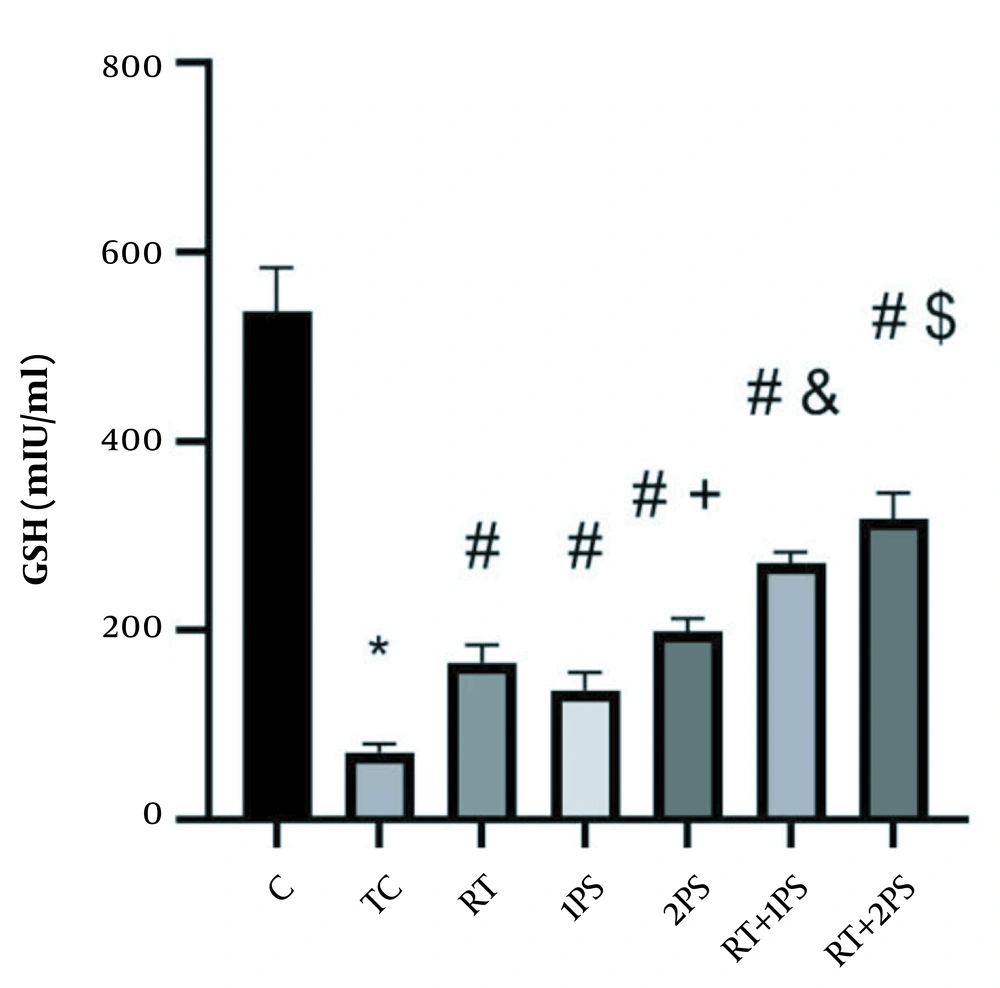
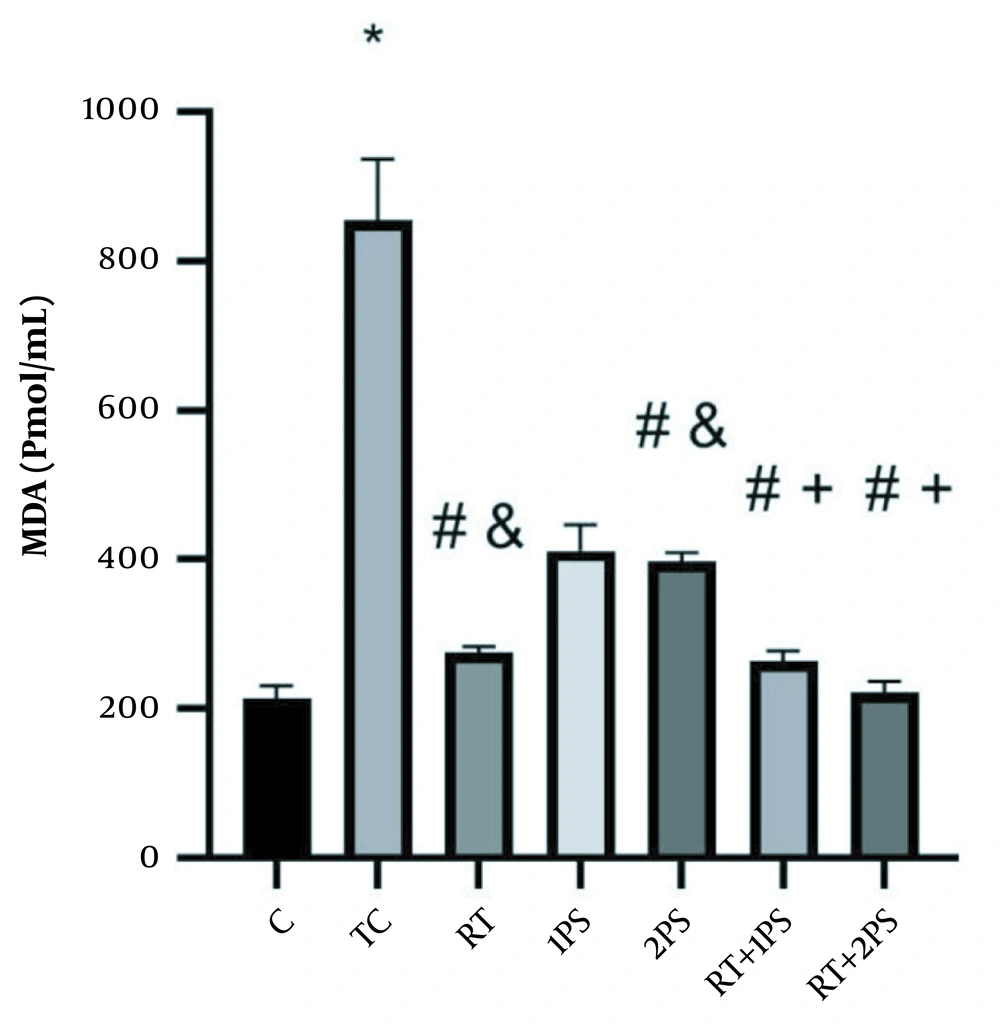
The levels of ATP, GSH, MDA, and PAB of rats are presented in Figures 1-4, respectively. There were significant differences in ATP (P = 0.001), GSH (P = 0.001), MDA (P = 0.001), and PAB (P = 0.001) levels in the heart tissue of the rats in seven groups of study. Tukey’s test showed that ATP (P = 0.001) and GSH (P = 0.001) levels in the TC group were significantly lower than the C group; nevertheless, MDA (P = 0.001) and PAB (P = 0.001) levels in the TC group were significantly lower than the group C.
ATP levels in the rats’ heart tissues. *, Significant decrease compared with C group; #, Significant increase compared with TC and RT groups; +, Significant increase compared with RT and 1PS groups; a significant increase compared with RT, 1PS, 2PS, and RT + 1PS groups; C, control; TC, treatment control; RT, resistance training, and PS, pumpkin seed.
GSH levels in the rats’ heart tissues. *, Significant decrease compared with C group; #, Significant increase compared with TC group; +, Significant increase compared with 1PS group; &, Significant increase compared with RT, 1PS, and 2PS groups; $, Significant increase compared with RT, 1PS, 2PS, and RT + 2PS groups; C, control; TC, treatment control; RT, resistance training; and PS, pumpkin seed.
MDA levels in the rats’ heart tissues. *, Significant increase compared with C group; #, Significant decrease compared with TC group; &, Significant decrease compared with 1PS; +, significant decrease compared with RT, 1PS, and 2PS groups; C, control; TC, treatment control; RT, resistance training; and PS, pumpkin seed.
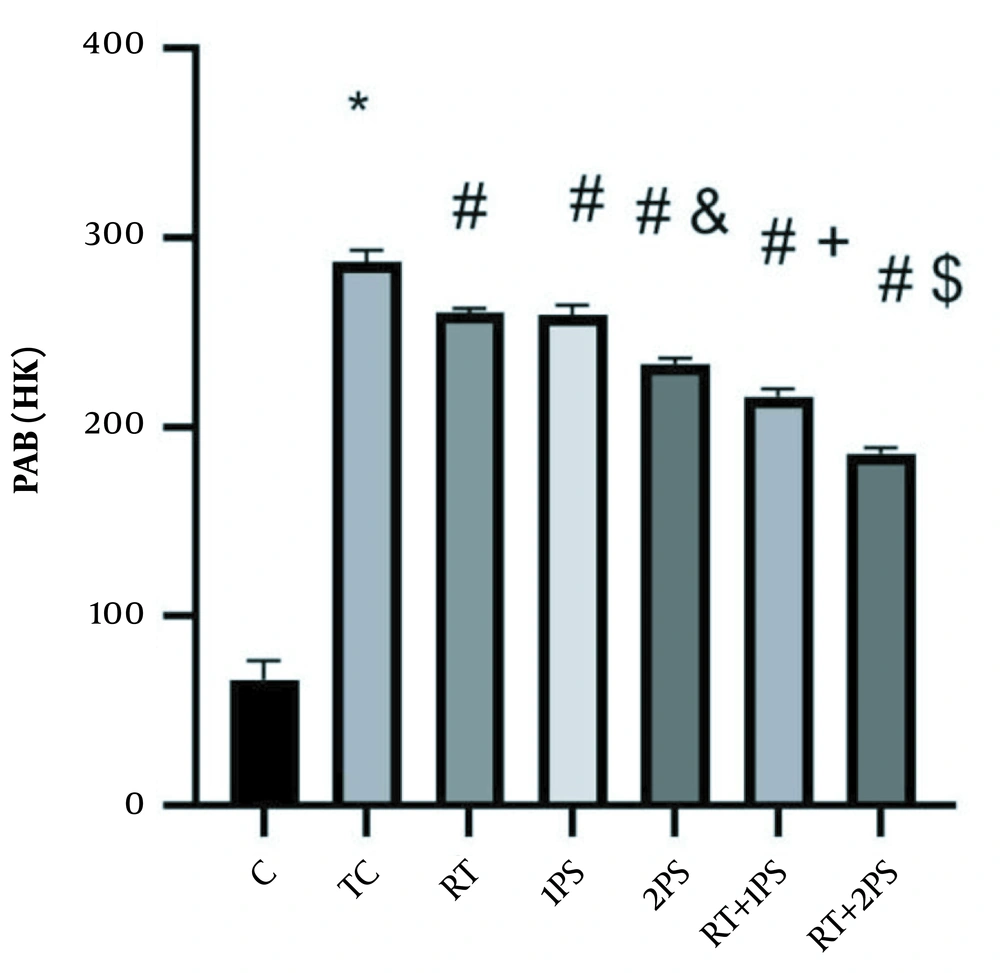
PAB levels in the rats’ heart tissues. *, Significant increase compared with C group; #, Significant decrease compared with TC group; &, Significant decrease compared with RT and 1PS groups; +, Significant decrease compared with RT, 1PS, and 2PS groups; $, Significant decrease compared with RT, 1PS, 2PS, and RT + 1PS groups; C, control; TC, treatment control; RT, resistance training; and PS, pumpkin seed.
ATP levels in 2PS (P = 0.004), RT + 1PS (P = 0.001) and RT + 2PS (P = 0.001) groups were significantly higher than the TC group; in the 2PS (P = 0.01), RT + 1PS (P = 0.001) and RT + 2PS (P = 0.001) groups were significantly higher than the RT group; in the RT + 1PS group were significantly higher than the RT (P = 0.001) and 1PS (P = 0.007) groups as well as in RT + 2PS group were significantly higher than the RT (P = 0.001), 1PS (P = 0.007), 2PS (P = 0.001), and RT + 1PS (P = 0.001) groups.
Moreover, GSH levels in the RT (P = 0.001), 1PS (P = 0.001), 2PS (P = 0.001), RT + 1PS (P = 0.001), and 2PS (P = 0.001) groups were significantly higher than the TC group; in the 2PS group were higher than the 1PS group (P = 0.001); in the RT + 1PS group were higher than the RT (P = 0.001), in the 1PS (P = 0.001), 2PS (P = 0.001), RT + 2PS groups were higher than the RT (P = 0.001), 1PS (P = 0.001), 2PS (P = 0.001) and RT + 1PS (P = 0.001) groups.
Also, MDA levels in RT (P = 0.001), 2PS (P = 0.001), RT + 1PS (P = 0.001), and 2PS (P = 0.001) groups were significantly lower than the TC group; in the RT (P = 0.001) and 2PS (P = 0.005) groups were significantly lower than the 1PS group; in the RT + 1PS and RT + 2PS groups were significantly lower than the RT (P = 0.001), 1PS (P = 0.001), and 2PS (P = 0.001) groups.
In addition, PAB levels in the RT (P = 0.001), 1PS (P = 0.001), 2PS (P = 0.001), RT + 1PS (P = 0.001), and RT + 2PS (P = 0.001) groups were significantly lower than TC group; in the 2PS group were significantly lower than the RT (P = 0.001) and 1PS (P = 0.001) groups; in the RT + 1PS group were significantly lower than the RT (P = 0.001), 1PS (P = 0.001), and 2PS (P = 0.001) groups. Besides, PAB levels in the RT + 2PS group were significantly lower than RT (P = 0.001), 1PS (P = 0.001), 2PS (P = 0.001), and RT + 1PS (P = 0.001) groups.
5. Discussion
The results of this study showed that H2O2-induced oxidative damage significantly reduced ATP and GSH, while increased MDA and PAB levels in the heart tissues of the rats; However, eight weeks 1PS significantly increased GSH, whereas decreased PAB levels. Also, 2PS significantly increased ATP and GSH levels, while decreased MDA and PAB levels in the rats’ heart tissues exposed to H2O2-induced oxidative damage. Consistent with the present study, 40 mg/kg PS oil in albino rats significantly reduced MDA, while increased GSH levels in methotrexate-exposed rats (17). Also, PS significantly decreased MDA, while increased catalase, SOD, and GSH in diabetic rats (18). Doses of PS administration can be the reason for consistent of noted (17, 18) findings with the results of the present study. In the present study, 2PS compared to 1PS had more effect on increased GSH levels as well as decreased MDA and PAB. It seems that the effects of PS consumption are dose-dependent. Of properties of PS oil is its dark green color, which is due to the presence of abundant chlorophyll and carotenoid pigments. This oil is also rich in oleic acid and essential fatty acid linoleic acid. The major sterol in this oil is delta-7-stenol, and its tocopherol is predominantly gamma tocopherol, which has an antioxidant role (19). It has been reported that ROS such as hydrogen peroxide can cause cell death through mechanisms such as lipid peroxidation, altering cellular proteins, and initiating various pathways to trigger stress messages (20). In contrast, phenolic compounds in the PS extract have been shown to suppress the lipid peroxidation pathway and inhibit the activity of free radicals (19).
In the present study, RT significantly increased GSH as well as decreased MDA and PAB levels in the rats’ heart tissue exposed to H2O2-induced oxidative damage. In line with the present study, high-intensity interval training (HIIT) significantly increased catalase and decreased MDA in rats (12). Also. RT significantly decreased MDA in the heart tissues of rats (21); however, 12 weeks of RT at an intensity of 70% of one maximal repetition (1RM) did not significantly alter MDA levels in male rats (22). Different statistical samples can be the reason for inconsistent findings of Rahbar and Ahmadiasl (22) study with the present study. Rahbar and Ahmadiasl (22) investigate the effect of RT in healthy rats, but in the present study, the researchers used rats exposed to H2O2-induced oxidative damage. According to the reported studies, the intensity of exercise seems to be an important factor affecting antioxidant enzymes changes, so that in most studies, whose findings indicate the antioxidant effects of exercise, the intensity of exercise implicated is high. It should be noted, however, that antioxidant responses to acute and intense physical activity are different from long-term physical activity, so that acute and intense physical activity may increase oxidative stress, but regular and long-term exercise activities will decrease oxidative stress by increasing antioxidant defense (23).
Concerning the interactive effects, in the present study, RT + 1PS and RT + 2PS significantly increased ATP and GSH, while decreased PAB levels. Also, RT + 2PS had more effect on the increase of ATP and decrease of MDA and PAB compared with RT, 1PS, 2PS, and RT + 1PS; therefore, the protective effects of RT simultaneously with PS administration appear to be greater than RT and PS alone. In this regard, pumpkin and its seeds have been reported to contain a high percentage of unsaturated fats, proteins, vitamins A and E, as well as the number of antioxidants such as lipophilic tocopherols and polar compounds (19). Tocopherols are the major lipophilic antioxidants in PS and oils. The seeds of this plant contain significant amounts of vitamin E derivatives, including tocopherols and tocotrienols (19). Regarding the effects of exercise activities, the studies reported show that exercise activities alter the antioxidant enzymatic activity of the tissues according to the type, duration, and intensity of the exercise. Therefore, in the present study, it seems that PS consumption and RT with different mechanisms have resulted in improved antioxidant status in the rats’ heart tissue exposed to H2O2- induced oxidative damage. Inability to evaluate the histological and pathological changes of heart tissue and to measure the amount of phenol and total antioxidant of PS extract were the limitations of the present study. Future studies are suggested to measure the histological and pathological changes in heart tissue of rats exposed to H2O2-induced oxidative damage in order to confirm the findings of the present study.
5.1. Conclusions
According to the results, it seems that concurrent resistance training and pumpkin seed consumption has protective interactive effects on heart tissue of rats exposed to H2O2-induced oxidative damage. Moreover, and the effects of pumpkin seed consumption are dose-dependent.