1. Background
Excessive usage of antibiotic is destructive to human health, ecosystem, and environment. It could also increase the incidences of drug-resistant pathogens [1]. Antibiotics resistance is a worldwide major problem which is rapidly increasing in both hospitals and the community involved in morbidity, mortality, and health-care [2].
Almost in all pathogenic bacteria, it has been observed that they are able to obtain the resistance factor to the antimicrobial drugs quickly, therefore, multiple drug resistant bacteria caused the main failure in the treatment of infectious diseases [2, 3]. So, it is necessary to search and design the alternative approaches to control resistant bacteria. One of the possible strategies is rational localization of bioactive phytochemicals with antibacterial activity [1, 4]. Currently, researchers have investigated plants with extensive variety of secondary compounds that could be a potential source for various antimicrobial agents [5, 6]. Those plants contain numerous structurally unique bioactive compounds which are decent sources to obtain natural therapeutic agents [7]. Coriander, parsley, oleander, myrtle, mint, henna, Aloe vera, christ’s thorn, olive, chamomile, cinnamon, licorice, and ginger are some of examples of promising species of medical plants [8].
Coriander (Coriandrum sativum): coriander is rich in vitamins, decanal, nonanal, linalool and many useful substances. It is active against almost all Gram positive and negative bacteria [1, 9]. Parsley (Petroselinum crispum): The herb and root are regularly suggested in traditional medicine for their alleged valuable impacts on gastric, menstrual, and urinary disorders, cough, and myalgia [10]. Oleander (Nerium oleander) is a large glabrous evergreen shrub with milky juice. The inhibitory effect of oleander leaf on certain Gram positive and Gram negative bacteria has been recognized as well [11].
Myrtle (Myrtus communis): Myrtus species are reported as very rich in volatile oils phenolic acids as gallic and ellagic acids, flavonoids, fatty acids (FA), tannins and anthocyanin pigments [12]. Mint (Mentha piperita): Menthol is popular for its disinfectant feature with effective antimicrobial properties that its impact was proved against 21 pathogen microorganisms [13]. Henna (Lawsonia inermis): is broadly used for medical and cosmetic purposes over the centuries. Henna’s bark, leaves, and seeds are used in medicine due to the high amount of phenolic compounds such as flavanol, Lawsone, tannin, gallic acid, glucose, mannitol, fat, resin, mucilage, and phenolic acid [14]. Aloe Vera: its leaves are source of great biologically active compounds, such as anthrones, anthraquinones, and various lectins. Aloe vera has been showed potential antifungal, antiviral and antibacterial activity against skin infections, such as acne, herpes and scabies [15]. Christ’s thorn (Ziziphus spina christi) It has been called as “Sedr” in Iran. Flavonoids, alkaloids, triterpenoids, saponins, lipids, proteins, free sugar, and mucilage are the main important compounds characterized in this plant [16]. Olive (Olea europaea): olives contain high concentrations of phenolic compounds. The main types of phenolic compounds present in olives include phenolic acids, phenolic alcohols, flavonoids, and secoiridoids. Hydroxytyrosol and tyrosol are the most abundant phenolic alcohols in olives [17]. Cinnamon (Cinnamomum zeylanicum): The procyanidine polyphenol from various natural sources are reported to offer strong protection against oxidative stress to primary glial cells [18]. Licorice (Glycyrrhiza glabra): more than 80 different ingredients of liquorice preparations have been known such as flavonoids, chalcones, and coumarone. The core biologically active compound of the liquorice root is glycyrrhizic acid or glycyrrhizin [19].
The present study aimed to evaluate antimicrobial effects of selected plants and combination of them against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Salmonella enteric isolates. These bacteria are resistant to the most common antibiotics [20].
2. Methods
2.1. Water Extracts Distillation
Coriander and parsley provided seeds; oleander, myrtle, mint, henna, aloe vera, christ’s thorn and olive provided leaves; chamomile flowers; cinnamon bark; licorice; and ginger roots were collected from Iranian research organization for Science and technology. By early washing, additions and dirties were removed and then plants were washed by distilled water. After that, plants were placed in dryer oven for two days. Dried plants were grinded and prepared for extraction. The aqueous extract was pulled out with Soxhlet apparatus. From each plant, 40 gram of powder was placed in filter paper bags, and water-soluble extract in 400 mL of distilled water was removed. The dilute extracts were poured in evaporator to remove the excessive water at temperature of 40°C. Then, samples were centrifuged (3000 rpm, 5 minutes) to eliminate the impuritiesand suspended solids. The supernatants were used as aqueous crude extract in this study.
2.2. Hydro-Alcoholic Extraction
The plants powders were prepared as explained in previous section. For hydro-alcoholic extraction, the powders were placed in flasks individually. Water and alcohol at a ratio of 50:50 in specified deal were added to the flasks and kept 24 hours in dark. Samples were poured in rotary evaporator to remove as much as possible extra water and alcohol. Then, the concentrated samples were centrifuged (3000 rpm, 5 minutes) and supernatants were used as hydro-alcoholic crude extract in this study.
2.3. Dry Weight Determination of Aqueous and Hydro-Alcoholic Plant Extracts
The weight of a plate was measured, and then 5 mL of each plant extracts (aqueous and hydro-alcoholic) were poured into the plates individually. The contents of the plate were dried at oven. On average, three replicates of weight differences were considered as the dry weight of the extract [21].
2.4. Bacterial Culture
Staphylococcus aureus (ptcc 1764), Escherichia coli (ptcc 1399), Pseudomonas aeruginosa (ptcc 1310), and Salmonella enteric sub specie (ptcc 1709) were purchased from the Persian type culture collection (PTCC), IROST, Iran. Bacteria were cultured in brain heart infusion (BHI) medium (Sigma-Aldrich) and used for assays [22, 23].
2.5. Zone of Inhibition Test
The antibacterial assay was based on the standard agar diffusion assay. One colony of each bacterium was picked off from a stock plate and suspended in deionized water individually. An aliquot of bacterium suspension was swabbed on agar plates (BHI-agar).Then, six holes were perforated in each plate and 100 µL of the same concentration of each plant extracts were poured in the hole. The plates were incubated at 37°C for 24 hours and then the diameter of the growth inhibition around each hole was measured [24].
2.6. Minimum Inhibitory Concentration (MIC) Test
The plant extracts that had a better inhibition zone were selected and used for MIC test. Half McFarland concentrations (OD620nm = 0.1) of each bacterium was cultured in both BHI medium in a universal bottle and five concentrations of selected plant extracts (10, 20, 30, 40 and 50 mg/mL), and penicillin (10, 20, 30, 40 and 50 µg/mL) was individually added to the bottles. In addition, 30 µg/mL of gentamicin was used for Gram negative bacteria alongside with different concentration of penicillin. The controls were contained only half McFarland concentrations (OD620nm = 0.1) of each bacterium. The bottles were incubated at 37°C for 24 hours. The OD620nm of the samples was recorded at the end of incubation. MIC was considered as the lowest concentration of the sample preventing visible growth, which OD620nm less than 0.2 was considered to have no visible bacterial growth. All samples were examined in three separate experiments [1].
2.7. Synergistic Effect
To study the synergistic effect, extracts with higher antibacterial effects were selected, including hydro-alcoholic extracts of myrtle and chamomile and water extract distillation of henna, aloe vera, christ’s thorn and cinnamon. Four treatments of equally mixed plant extracts were tested on S. aureus and P. aeruginosa [25].
2.8. Statistical Analysis
Data were analyzed with a statistical software program (SPSS 16). Comparisons between multiple numeric datasets were performed using one-way ANOVA followed by Duncan multiple-range test. Results are expressed as mean ± SEM., and statistical significance was accepted at P < 0.05.
3. Results
3.1. Antimicrobial Activity of Plant Extracts
The results showed that the most active organic solvent to extract the antibacterial compounds from tested plants was ethanol. As shown in Table 1, water extract distillation of parsley, oleander, olive, licorice, and ginger could not inhibit the growth of tested bacteria while hydro-alcoholic extracts of them prevented the growth of some and/or all of the tested bacteria (hydro-alcoholic extract of ginger).
| Plant Part Used | Plant Extract | S. aureus | E. coli | P. aeruginosa | S. enteric |
|---|---|---|---|---|---|
| Seed | Aqueous Coriander | 15 | 0 | 0 | 0 |
| Hydro-alcoholic Coriander | 15 | 0 | 0 | 0 | |
| Aqueous Parsley | 0 | 0 | 0 | 0 | |
| Hydro-alcoholic Parsley | 0 | 10 | 0 | 0 | |
| Leaf | Aqueous Oleander | 0 | 0 | 0 | 0 |
| Hydro-alcoholic Oleander | 0 | 0 | 0 | 12 | |
| Aqueous Myrtle | 15 | 0 | 0 | 20 | |
| Hydro-alcoholic Myrtle | 28 | 13 | 15 | 14 | |
| Aqueous Mint | 0 | 17 | 15 | 17 | |
| Hydro-alcoholic Mint | 19 | 0 | 0 | 0 | |
| Aqueous Henna | 20 | 18 | 10 | 10 | |
| Hydro-alcoholic Henna | 18 | 0 | 0 | 0 | |
| Aqueous Aloe vera | 17 | 0 | 20 | 20 | |
| Hydro-alcoholic Aloe vera | 0 | 0 | 0 | 0 | |
| Aqueous Christ’s thorn | 0 | 15 | 16 | 14 | |
| Hydro-alcoholic Christ’s thorn | 10 | 0 | 0 | 0 | |
| Aqueous Olive | 0 | 0 | 0 | 0 | |
| Hydro-alcoholic Olive | 13 | 0 | 0 | 10 | |
| Flower | Aqueous Chamomile | 10 | 10 | 0 | 0 |
| Hydro-alcoholic Chamomile | 17 | 13 | 10 | 10 | |
| Bark | Aqueous Cinnamon | 23 | 0 | 14 | 14 |
| Hydro-alcoholic Cinnamon | 16 | 0 | 0 | 0 | |
| Root | Aqueous Licorice | 0 | 0 | 0 | 0 |
| Hydro-alcoholic Licorice | 0 | 0 | 0 | 22 | |
| Aqueous Ginger | 0 | 0 | 0 | 0 | |
| Hydro-alcoholic Ginger | 18 | 13 | 15 | 15 | |
| Control | Penicillin* | 32 | 20 | 20 | 18 |
aThe data are in millimeter and recorded after 24 hours incubation of agar plates at 37°C. The data are expressed as the mean.
bA combination of penicillin with 30 µg/mL of gentamicin was used against E. coli and P. aeruginosa.
The hydro-alcoholic extracts of myrtle, chamomile, and ginger were able to inhibit the growth of all tested bacteria while only aqueous extract of henna showed the inhibitory effect against all bacteria. The highest zones of inhibition were recorded for the hydro-alcoholic extract of myrtle followed by aqueous extract of cinnamon and hydro-alcoholic extract of licorice, which were 28, 23, and 22 mm in diameters, respectively. In addition, between the four tested bacteria, the growth of S. aureus was more effected by the plant extracts followed by S. enteric, P. aeruginosa and E. coli.
The growth of S. aureus was inhibited by the aqueous extraction of cinnamon, henna, Aloe vera, coriander, myrtle and chamomile and hydro-alcoholic extraction of myrtle, mint, henna, ginger, chamomile, cinnamon, coriander, olive, and christ’s thorn by displaying the zone of inhibition between 10 to 23 mm and 10 to 28 mm, respectively.
Moreover, among different herbal extracts, aqueous extraction of henna, mint, christ’s thorn, and chamomile as well as hydro-alcoholic extraction of myrtle, chamomile, ginger, and parsley had antibacterial effects on E. coli by exhibition the zone of inhibition between 10 to 18 mm and 10 to 13 mm, respectively.
Moreover, aqueous extraction of Aloe vera, christ’s thorn, mint, cinnamon, henna and hydro-alcoholic extraction of myrtle, ginger, and chamomile inhibited the growth of P. aeruginosa by presenting the zone of inhibition size between 10 to 20 mm and 10 to 15 mm, respectively.
Furthermore, aqueous extraction of myrtle, Aloe vera, mint, christ’s thorn, cinnamon, henna and hydro-alcoholic extraction of licorice, ginger, myrtle, oleander, olive, and chamomile repressed the growth of S. enteric by demonstrating the zone of inhibition between 10 to 20 mm and 10 to 22 mm, respectively.
Among all four bacteria, only S. enteric exposed more sensitivity to hydro-alcoholic extract of licorice and aqueous extracts of myrtle and Aloevera compared to penicillin (22, 20 and 20 mm vs. 18 mm, respectively). Also, P. aeruginosa showed similar sensitivity to aqueous extract of Aloe vera compared to penicillin (20 mm vs. 20 mm, respectively).
3.2. Minimum Inhibitory Concentration (MIC)
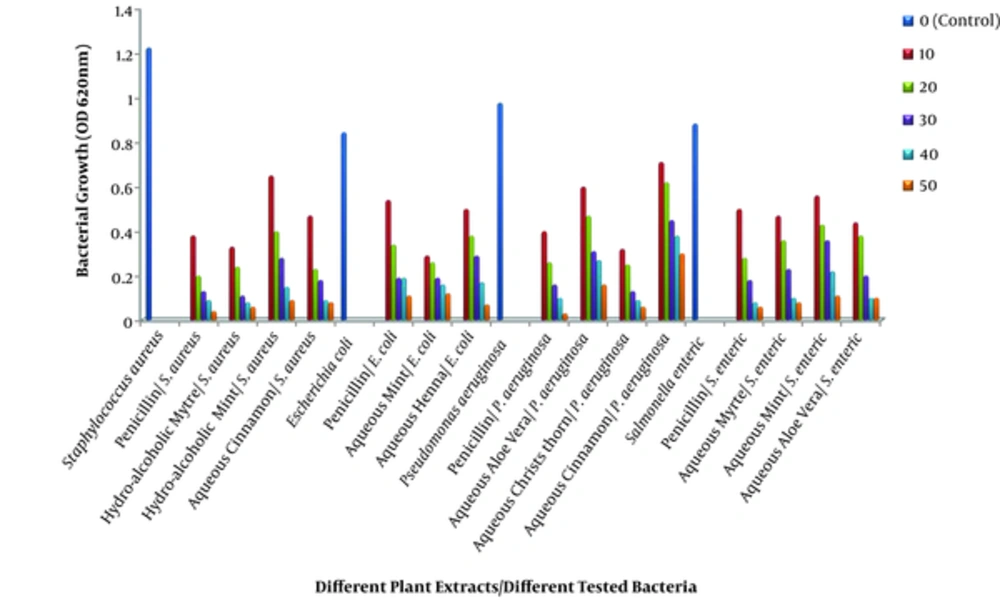
The minimum inhibitory concentration (MIC) was studied on eight different plant extracts (including both aqueous and hydro-alcoholic extracts) using different concentration against different bacteria (Figure 1). The OD620nm of bacterial growth contained the plant extracts compared with growth of bacterial culture, which contained no extracts. Moreover, for each bacterium, different concentration of penicillin was tested as the control. The turbidity of the cultures with the OD620nm less than 0.2 was not visible. The results showed that all extracts could inhibit the growth of all four tested bacteria but with different sensitivity. The MIC of penicillin against different tested bacteria was 30 µg/mL. Among the plants tested, hydro-alcoholic extract of myrtle and aqueous extracts of cinnamon, mint, christ’s thorn, and Aloe vera showed strong activity against S. aureus (for the first two extracts), E. coli, P. aeruginosa, and S. enteric with the best MIC at 30 mg/mL, respectively. In addition, the MIC values of hydro-alcoholic extract of mint and aqueous extract of henna and myrtle to inhibit.
Concentration used for plant extract was mg/mL and for penicillin was µg/mL. Samples were incubated at 37°C for 24 hours. Culture bacteria containing no plant extract or antibiotics were considered as control. The data are expressed as the mean. Note: A combination of different concentration of penicillin with 30 µg/mL of gentamicin was used against E. coli and P. aeruginosa.
The growth of S. aureus, E. coli and S. enteric were 40 mg/mL, respectively. The MIC of aqueous Aloe vera and mint against P. aeruginosa and S. enteric were 50 mg/L respectively. While, 30 mg/mL of aqueous extract of cinnamon inhibited the growth of Gram-positive S. aureus; the MIC of this extract against Gram negative P. aeruginosa was higher than 50 mg/mL.
3.3. Synergistic Effect
To study the synergistic effect, few extracts were chosen based on the results of the first experiment, involving hydro-alcoholic extracts of myrtle and chamomile and aqueous extracts of henna, Aloe vera, Christ\s thorn and cinnamon. Four different combinations of effective herbal extracts were tested to overcome the resistance of Gram-positive (S. aureus) and Gram-negative (P.aeruginosa) bacteria (Table 2). Results showed that combination of myrtle, henna, and Aloe vera were more effective than the other treatments by displaying the inhibition zone of 15 and 21 mm against the growth of P. aeruginosa and S. aureus, respectively. However, the result of the first experiment showed myrtle, henna and Aloe vera individually caused 28, 20, and 17 mm diameter of inhibition zone on S. aureus culture. Therefore, it can be concluded that theses extracts contained compounds which presented antagonist effect on growth inhibition of tested bacteria. The same effect was observed to study the combination of extracts on P. aeruginosa even lower diameter of inhibition zone on P. aeruginosa culture was detected.
| Treatment Combination | S. aureus | P. aeruginosa |
|---|---|---|
| Myrtle, Henna, Aloe vera | 21 | 15 |
| Myrtle, Henna, Aloe vera, Chamomile, Christ’s thorn | 15 | 15 |
| Myrtle, Henna, Aloe vera, Chamomile, Christ’s thorn, Cinnamon | 19 | 14 |
| Chamomile, Christ’s thorn, Cinnamon | 18 | 14 |
aThe data are in millimeter and recorded after 24 hours incubation of agar plates at 37 °C. The data are expressed as the mean.
4. Discussion
Increasing the number of multi-drug resistance pathogenic microbes in human and animal as well as unwanted side effects of certain antibiotics has encouraged enormous interest to search for new antimicrobial drugs of plant origin [26].
All of the four tested bacteria in this study approximately responded to water extract distillation and hydro-alcoholic extracts with greater result for hydro-alcoholic extract. However, it has been reported by many researchers that hydro-alcoholic extract, compared to the aqueous extract, is more effective and has a superior inhibitory influence [27].
The present investigation showed that water extract distillation and hydro-alcoholic extract of coriander had no effect on Gram-negative bacteria. However, Kubo et al. [28] showed positive effect of coriander on Salmonella, which is a Gram-negative bacterium. Antibacterial activity of coriander is due to the presence of alpha, beta-unsaturated aldehydes [29]. In a survey, Toroglu [30] reported the inhibitory effect of coriander on different Gram-positive and Gram-negative bacteria. In addition, Lo Cantore et al. [31] considered antimicrobial properties for coriander in their study. Another study reported that volatile compounds of coriander could possess bactericidal activity against Salmonellacholera [32].
Dorman et al. [10] pointed out that parsley, in addition to antimicrobial effect, has remarkable antioxidant effect because it contains carotenoids, coumarins, flavonoids, tannins and triterpenes. Manderfeld et al. [33] suggested that anti-microbial effects of parsley is related to the presence of furocoumarins compound in this plant. However, in the present research, both water extract distillation and hydro-alcoholic extract of parsley did not show appropriate antibacterial properties.
In this study, S. aureus showed more sensitivity to most of aqueous and hydro-alcoholic plant extracts, but the different effect was observed in aqueous extracts of mint and christ’s thorn, which only inhibited the growth of all Gram-negative tested bacteria. Only 10 mg/mL of the aqueous extracts of mint and christ’s thorn inhibited the growth of E. coli and P. aeruginosa to less than 35% of the control. However, the hydro-alcoholic extracts of these two plants could only inhibit the growth of S. aureus and not the Gram-negative bacteria. According to Abouhosseini Tabari et al. [13], mint essence had a weak effect on both Gram-negative and positive bacteria (E. coli and S. aureus, respectively). One of the mint components is hydrophobic, which could disintegrate the bacterial cell wall and cause disruption in their structure and permeation. Sabahat et al. [34] investigated the effect of juice and essential oil of mint on several bacteria and observed antibacterial activity with 11.78 mm mean inhibition zone. In addition, in another study regarding the antimicrobial effects of mint essence, Aridogan et al. [35] confirmed the presence of antimicrobial agent against S. aureus and E. coli. Also, Iscan et al. [36] reported the significant inhibitory impact of mint extract against two Gram-positive (Bacillus subtilis and S. aureus) and two Gram-negative (E. coli and P. aeruginosa) bacteria. However, Shan et al., [37] reported that in vitro antibacterial activity test of mint extract showed wider diameter of inhibition zone on S. aureus culture compared to the E. coli, which was observed in the present study. Derwich et al. [21] reported that mostly aqueous plant extract was effective on Gram-negative and hydro-alcoholic extract was effective on Gram-positive bacteria such as christ’s thorn, which is a herb with thirty essential oils.
Myrtle and henna are two herbs with many bioactive compounds. Polyphenols are common compounds in myrtle and henna that have antioxidant and antibacterial effects [26]. Nevertheless, in the current study, the most antibacterial effect of myrtle was for hydro-alcoholic extract, while henna was affected by water extract distillation. In addition, 20 mg/mL of aqueous extracts of myrtle and henna prevented more than 50% of S. enteric and E. coli growth, respectively, while only 10 mg/mL of hydro-alcoholic extract of myrtle was able to reduce the growth of S. aureusto less than 25% compared to the control. By taking into account 20 of ethanol extracts plants species, which are Yemeni traditional herbals to treat infectious diseases, Ali et al. [38] found that ethyl acetate extract of henna was the most active antibacterial against all the bacteria in the test system. Moreover, Baba-Moussa et al. [39] indicated that water extract distillation of leaves of henna had the substantial antibacterial effect. Quinonic compounds from henna were studied in-vitro for antimicrobial properties. Genotoxic studies on lawsone (or hennotannic acid), which is a dye present in the leaves of the henna plant, showed a weak bacterial mutagen for Salmonella typhimurium strain TA98 and more clearly mutagenic for straining TA2637. However, Kirkland and Marzin [40] stated that the weight of evidence revealed henna possess no genotoxic risk to the consumer.
Similarly, Thakur et al. [41] reported that ethanolic extract of cinnamon was not effective on Gram-negative bacteria, but ginger prevented the growth of the bacteria. Cinnamon has many bioactive compounds including alkaloids, flavones, phenols, quinones, terpenoids, glycosides, and tannins known to possess antibacterial activity [41]. Selecting appropriate solvent to extract the antibacterial compound from plant is crucial because inhibition zone of water extract distillation of cinnamon in the present study was remarkable especially on S. aureus, which is similar to the study done by Buru et al. [42]. Only 10 mg/mL of the aqueous extract of cinnamon inhibited more than 40 % of the growth of S. aureus.
The same result with Nitalikar et al. [19] study showed that licorice extracts (both water distillation and hydro-alcoholic extracts) had no antibacterial activity except for its hydro-alcoholic extract, which could inhibit the growth of S. enteric by displaying a wide inhibition zone. Jastaniah [1] studied the proper antibacterial effect of phenolic compound of oleander and olive leaves, however, in the present research, only the hydro-alcoholic extracts of these two leaves could inhibit the growth of S. aureus and S. enteric.
Antibacterial activity of oleander on certain Gram-positive and Gram-negative bacteria was studied and considerable antimicrobial activity was found [11].The antimicrobial activity may be due to a wide variety of secondary metabolites, such as tannins, terpenoids, alkaloids, and flavonoids, which have antimicrobial activities [7].
Also, Aloe vera leaf was recognized as increasing collagen building, but its antibacterial effect was not negligible. Mannans, polymannans, anthraquinone c-glycosides, anthrones, anthraquinones, and various lectins are recognized as bioactive compound of Aloe vera [15]. In the present study, aqueous aloe vera extract demonstrated a good antibacterial activity against P. aeruginosa, S. enteric and S. aureus, and only 20 mg/mL of the Aloe vera extract inhibited more than 50% of P. aeruginosa and S. enteric growth.
Overall, all the plant extracts even at 10 mg/mL concentration inhibited the growth of tested bacteria compared to the control containing no extract. It can be found that the antimicrobial agents are presented in the extracts. However, the synergistic effect study showed that the mixture of these extracts could reduce their inhibitory effects. Abouhosseini Tabari et al. [13] found that synergistic effect was not observed in combination 1:1 of mint and eucalyptus (myrtle family) essence. Even this combination managed to reduce the antimicrobial activity and the inhibition was less than mint and eucalyptus essence individually. This result might be due to some components in mint and eucalyptus essences, which are antagonists and might neutralize each other and weaken their antimicrobial activity.
To sum up, Gram-negative bacteria show more resistance to the available antibiotics [43]. Comparative study on the cell wall structures of bacteria reveals that Gram-positive bacteria have thick peptidoglycan in their cell wall composition while Gram-negative bacteria have only a thin layer of peptidoglycan, but rich in lipoprotein and lipopolysaccharides in their cell structure. Thus, Gram negative bacteria are more resistant [43]. Hence, the effects of antimicrobial agent against Gram positives bacteria were more tangible than those against the Gram negatives. It seems obviously that the active compounds belong to the lipophilic group rather than to the hydrophilic one [26].
4.1. Conclusion
Plant extracts contained a very complex structure with the active ingredients present in the form of natural organic compounds. The process of extraction for a particular compound is dependent on the solubility of the component in the solvent (water or organic solvent). The process and extraction system are constantly different with every product and compound. The crude extracts of the tested plants demonstrated good potential antibacterial activities. The potential to develop antimicrobial compounds from higher plants appears rewarding as it will propel to the expansion of a phytomedicine to turn against multidrug resistant microbes.