1. Background
Tuberculosis (TB) is a global emergency and is amongst the worldwide health threats today. TB remains the number one killer infectious disease affecting adults in developing countries [1]. Over the last two decades current control efforts are severely hampered due to Mycobacterium tuberculosis is a leading opportunistic infection in patients with the acquired immune deficiency syndrome and also due to the spread of multidrug-resistant strains (MDR-MTB) [2]. Inorganic medicinal chemistry is recently one of the most fields of research. The most significant part of this research has been directed to the development of drugs active against M. tuberculosis [3-5]. Hydrazones belonging to azomethine class of compounds have attracted the attention of many chemists owing to their wide spectrum of pharmacological activity profile with structural flexibility and ligating behavior. Hydrazones distinguished by the presence of two interlinked nitrogen atoms R1CN-NR2 are interesting, because they can function as antimicrobial, antitubercular and antitumor agents [6-8]. Having been used as the cornerstone of anti-tuberculosis therapy for more than half a century, much has been learned about the biochemistry and multifaceted modes of action of the frontline drug isoniazid. Indeed, with the enormous global burden of tuberculosis and the alarming rise in the number of clinical isolates displaying drug resistance or increased virulence [9-12], isonicotinic acid hydrazide has become the single most researched anti tubercular agent [13-15]. Isonicotinoylhydrazide (Isoniazid: INH) is one of the most potent anti-TB drugs, used to kill the M. tuberculosis. Isonicotinoylhydrazone derivatives containing heterocyclic moiety have found to exhibit better anti-tubercular activity [16, 17]. We aimed to synthesize a potential ligand containing both, isonicotinoylhydrazide and 3-ethoxy-2-hydroxybenzilidine moieties, linked through azomethine group. Thus, the present investigation throw light on synthesis, characterization, antimicrobial and anti-tubercular activity of novel Schiff base ligand viz., N’-(3-ethoxy-2-hydroxybenzilidine) isonicotinohydrazide.
2. Objectives
We aimed to synthesize a potential ligand containing both, isonicotinoylhydrazide and 3-ethoxy-2-hydroxybenzilidine moieties, linked through azomethine group. Thus, the present investigation throw light on synthesis, characterization, antimicrobial and anti-tubercular activity of novel Schiff base ligand viz., N’-(3-ethoxy-2-hydroxybenzilidine) isonicotinohydrazide.
3. Materials and Methods
Physical measurements: In this descriptive study, NMR spectra were recorded at ambient temperature with a Bruker Avance 500 MHz spectrometer using DMSO as solvent, chemical shift values (δ) are given in ppm. Infrared spectra (4000 - 400 cm-1) were recorded as KBr discs with an IR Prestige-21 Shimadzu FT-IR spectrophotometer. Microanalyses (C, H, N) of the ligands were carried out on a Leco CHNS elemental analyzer.
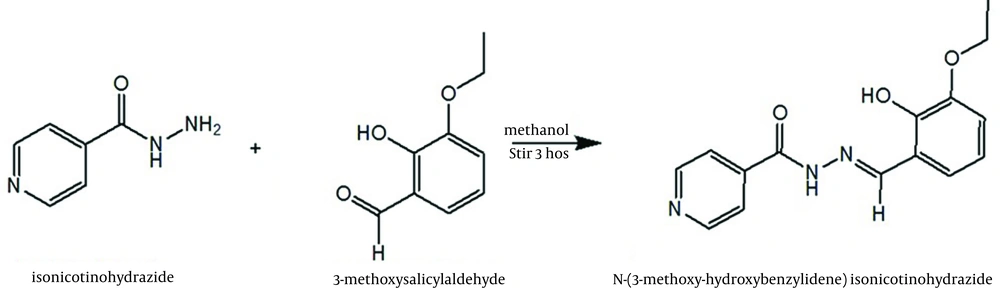
Synthesis of N’-(3-ethoxy-2-hydroxybenzilidine) isonicotinohydrazide ligand: 3-ethoxysalicylaldehyde (1.66 g, 0.01 mol) was added to a solution of isonicotinohydrazide (1.37 g, 0.01 mol) in methanol (30 mL) and stirred for 3 h. The pale yellowish solid separated was filtered, washed repeatedly with methanol, dried in air and recrystallized from ethanol (Figure 1). Yield: 93%.
Antibacterial activity: Compound was prepared by dissolving 1mg of sample in 10 mL of 2% DMSO to give the concentration 100 µg/mL. The standard solutions of Ceftazidime (antibacterial drug) and ceftriaxone (antifungal drug) were prepared in 2% DMSO to give concentration of 100 µg/mL. Serial broth micro dilution was adopted as a reference method. Serial dilutions of test compound were made in broth, after which a standardized microorganism suspension was added. Quantities of test ligand were serially diluted to attain the final concentrations of 512, 256, 128, 64, 32, 16, 8, 4, 2, and 1 µg/mL. One of the test tubes was kept as control. Each of the 10 test tubes was inoculated with a suspension of microorganism to be tested and incubated at 35ºC for 18 h. At the end of the incubation period, the tubes were visually examined for the turbidity. Cloudiness in the test tubes indicated that microorganism growth has not inhibited by the antibiotic contained in the medium at the test concentration. A serial dilution of test compound was made in broth, after which a standardized microorganism suspension was added. A quantity of test compound was serially diluted to attain the final concentrations of 512, 256, 128, 64, 32, 16, 8, 4, 2, and 1 µg/mL. Test tubes were inoculated with suspension of microorganism (1 × 106 CFU/mL) to be tested and incubated at 37ºC for 24 h. Cloudiness in the test tubes indicated that microorganism growth has not inhibited by the antibiotic contained in the medium at the test concentration.
Anti-tubercular activity: Test compound was evaluated for in vitro anti-mycobacterium activity. The MIC was determined and interpreted for M. tuberculosis H37Rv according to the procedure of the approved microdilution reference method of antimicrobial susceptibility testing [18]. Compound was taken at concentrations of 512, 256, 128, 64, 32, 16, 8, 4, 2, and 1 µg/mL. M. tuberculosis H37Rv strain was used in Middle brook 7H-9 broth which was inoculated with standard as well as test compound and incubated at 37ºC for 4 weeks. The bottles were inspected for growth twice a week for a period of 3 weeks. Reading was taken at the end of fourth week. The appearance with turbidity of 1 × 106 CFU/mL was considered as bacterial growth and indicates resistance to the compound. The growth was confirmed by making a smear from each bottle. Test compound was compared to reference drugs isoniazid (MIC = 0.025 µg/mL). The antimicrobial and anti-tubercular activity test was run in triplicate.
4. Results
The elemental analysis of ligand is in consistence with the molecular formula C15H15N3O3.
1H NMR (500 MHz, [D6] DMSO, 25ºC): δ = 12.27 (s, 1 H, -OH), 10.72 (s, 1 H, -NH), 8.69 (s, 1 H, -CHN), 8.78 [d, 3J (H, H) = 5.55 Hz, 2 H, H (11, 12)], 7.85 [d, 3J (H, H) = 5.95 Hz, 2 H, H (10, 13)], 7.17 [dd, 3J (H, H) = 7.75 Hz, 4J (H, H) = 0.95 Hz, 1 H, H (5)], 7.00 [dd, 3J (H, H) = 7.90 Hz, 4J (H, H) = 0.85 Hz, 1 H, H (3)], 6.82 [t, 3J (H, H) = 7.90 Hz, 1 H, H (4)], 4.04 [q, 3J (H, H) = 6.95 Hz, 2 H, -OCH2], 1.33 [t, 3J (H, H) = 6.95 Hz, 3 H, -CH3] ppm.
13C NMR (125 MHz) δ = 161.2, 150.3, 149.1, 147.5, 147.1, 139.9, 121.4, 120.7, 119.1, 118.9, 115.4, 84.1, 14.7 ppm.
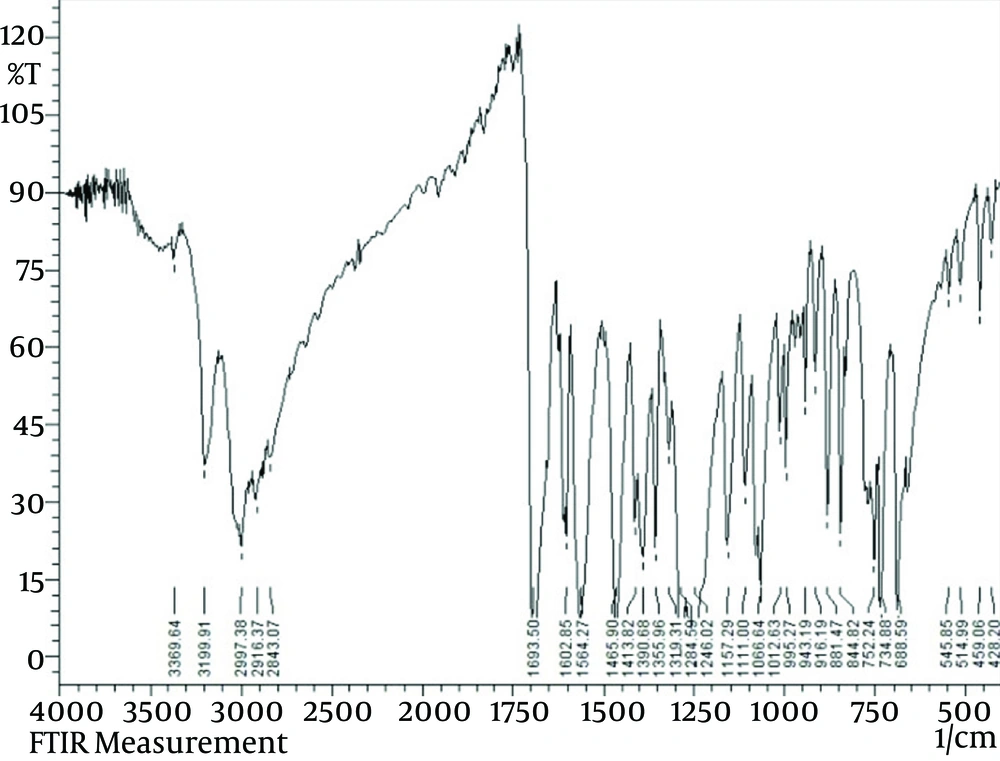
IR (KBr, cm-1); 3200 (νN–H); 1693 (νC = O); 1602 (νC = N); 1564, 1466, (νC = C); 1257 (νC-O); 995 (νN-N). Anal. Calc. for C15H15N3O3 (285.3) (%): C, 63.15; H, 5.30; N, 14.73. Found (%): C, 63.17; H, 5.23; N, 14.85.
The ligand show higher activity against SA (MIC 8 µg/mL) and EC (MIC 4 µg/mL) (Table 2).
The MIC of compound compared with isoniazid, standard anti tubercular drugs are summarized in Table 2. Lignad show inhibition at concentration 4 µg/mL.
5. Discussion
Ligand is soluble in solvents like DMF and DMSO but insoluble in common organic solvents. Anal. Calc. for C15H15N3O3: 285.3%; C: 63.15%; H: 5.30%; N: 14.73%. Found, C: 63.17%; H: 5.23%; N: 14.85%.
The IR band assignment of ligand is included in Table 1. IR spectrum of ligand is displayed in Figure 2. The coordination sites of ligand are determined using IR. The IR spectrum of the ligand exhibit two bands in the regions 3200 and 1693 cm-1 due to ν (NH) and ν (C = O) stretches. A new band appearing in the 1257 cm-1 is assigned to the ν (C-O) (enolic) mode. The band due to the imine group ν (C = N) mode of ligand at 1602 cm-1. In complex, the lactone carbonyl stretching has shifted to lower wave number by 32-75cm-1 indicating its involvement in coordination [19]. Also, shift of (N-N) band to the higher frequency region can be taken as an additional evidence for the participation of azomethine group incoordination [20]. Thus ligand functions as amonobasic tridentate ligand coordinating through lactone carbonyl oxygen, azomethine nitrogen and amide carbonyl oxygen via deprotonation.
| Functional Group | Characteristic Absorptions (cm-1) |
|---|---|
| 3200 | |
| 1693 | |
| 1602 | |
| 1246, 1157 | |
| 995 |
Diagnostic IR Bands (cm-1) of ACINH and its Complexes
Typical proton NMR spectra of the ligand was recorded at room temperature using DMSO as the solvent and the data was summarized in ‘‘Experimental’’ section. NMR of ligand has been analyzed in order to specify the coordination through lactone carbonyl oxygen and amide carbonyl oxygen. In the 1H NMR spectrum of ligand a signal at 12.27 ppm is assignable to the -OH proton, while a signal at 10.72 ppm is assigned to the -NH proton. Furthermore, azomethine proton is observed as a singlet at 8.69 ppm. Aromatic protons of the ligand appear well within the expected range. The aromatic protons of the ligand appeared at 6.81 - 9.79 ppm. Aromatic protons of the ligand appear well within the expected range. A quartet at 4.04 ppm [3J (H, H) = 6.95 Hz] is assigned to the -OCH2 protons, coupling with the -CH3 protons, while a triplet at 1.33 ppm [3J (H, H) = 6.95 Hz] is assigned to the -CH3 protons, coupling with the -OCH2 protons [19].
The prepared compound was screened for the inhibition of microbial growth under standard conditions, which may be utilized for demonstrating their antimicrobial efficacy using a serial broth microdilution method in 96 multi-cell micro liter plates [21]. Compound is tested at different concentrations and minimum inhibitory concentration (MIC) is determined. The antibacterial activity of synthesized compound is tested against bacteria like S. aureus (SA, ATCC 9144), E. coli (EC, ATCC 87261) and result is included in Table 2. The antibacterial activity of synthesized compound is compared with standard antibacterial drug. The results are compared with standard antibacterial drug ciprofloxacin and ceftriaxone. The ligand show higher activity against SA (MIC 8 µg/mL) and EC (MIC 4 µg/mL) (Table 2).
| Compound | Gram positive bacteria ( | Gram negative bacteria ( | Mycobacterium tuberculosis ( |
|---|---|---|---|
| 8 | 4 | 4 | |
| 0.010 | 0.015 | - | |
| 0.015 | 0.020 | - | |
| - | - | 0.025 |
In Vitro Antimicrobial Activity of the Compound and Standard Drug (MIC In µg/mL)
As the ligand is a derivative of isoniazid, ligand was tested for anti-tubercular activity in order to compare with the activity of isoniazid (Table 2).
The anti-mycobacterium activity of the synthesized compound is assessed against M. tuberculosis H37RV at 512, 256, 128, 64, 32, 16, 8, 4, 2, and 1 µg/mL. The MIC of compound compared with isoniazid, standard anti tubercular drugs are summarized in Table 2. Ligand shows inhibition at concentration 4 µg/mL. The Schiff bases ligand was readily prepared for evaluation against M. tuberculosis in good yield. Compound show strong level of activity in vitro [22, 23].
Therefore, the present study describes the preparation of N’-(3-ethoxy-2hydroxybenzilidine) isonicotinohydrazide. 1H and 13C NMR studies of ligand confirm the formation of title compound. Compound acts as monobasic, tridentate ligand. On the basis of this observation, structure is tentatively. In vitro anti-microbial and anti-tubercular activity of synthesized compound show good results enhancement of activity.