1. Background
Cyclophosphamide is an anticancer drug, which is used in chemotherapy. It causes alkylation of the DNA molecule. It is absorbed well by gastrointestinal tract and distributed extensively throughout the tissues and liquids of body. It also passes through the blood–brain barrier. It is turned into the active metabolites in liver and eventually excreted through the kidneys [1]. Cyclophosphamide applies its cytocidal effect through creating links between the double-stranded DNA molecules, breaking DNA and RNA, and inhibiting protein synthesis. It is an alkylating drug and creates reactive molecules that alkynate the nucleophilic group on DNA arm, especially guanine-N7. It creates lateral links between arms, the abnormal coupling of arms, and break in DNA molecule [2]. Consuming cyclophosphamide often follows anorexia, nausea and vomiting. Its major side effects include reduced blood cells, elevated concentrations of uric acid, decreased gonadal function, causing amenorrhea, azoospermia, and oligospermia [1, 2]. Medicinal plants have been used for many years throughout the world for treatment and prevention of certain diseases; however, the effects of some of them have been studied scientifically. Medicinal plants are beneficial therapies both in modern and traditional systems. Allium species such as onion, garlic are consumed as foods, spices, seasonings, and local drugs. Garlic (scientific name: Allium sativum from Liliaceae family) has considerably gained attentions among new plants, as it is extended throughout the world and it is available [3]. Compounds of garlic are many various as C, A, B6, B2, B1, alicine, sulfur, ajoine [3]. Biological responses of garlic including reduced cardiovascular risk factors, cancer, stimulation of immune activities, increased detoxification of foreign compounds (xenobiotic), anti-microbial and anti-viral effect, antioxidant activity, protection of the generated cells in adult male rats, which reflects its antioxidant role, protected sperm and caused final maturation and sperm evolution [3-5]. As the therapeutic and prophylactic effects of garlic on reproduction after consuming cyclophosphamide have not been studied yet, we attempted to study the effect of garlic extract on changes of serum concentration of LH, FSH hormones, testosterone, and spermatogenesis. As the chemical drugs, especially chemotherapy drug of cyclophosphamide decrease spermatogenesis, and reduce reproductive activity, and garlic contains antioxidant compounds, inhibitors of active metabolites, and free radicals, it can be partially predicted that garlic extract compounds reduce the damaging effects of cyclophosphamide on reproduction process [1, 3].
2. Objectives
The aim of this study was the effect of hydro-alcoholic garlic extract on change sexual hormones LH, FSH, testosterone in mature male rats under chemotherapy with cyclophosphamide.
3. Materials and Methods
In this experimental study, Allium sativum samples were obtained from the Agricultural and Natural Research Center of Fars province. Identification of genus and species samples was done by plant taxonomy experts from the College of Science/Shiraz University. Hydro alcoholic extracts of Allium sativum were obtained using the method of Erdemoglu et al. [6]. This research was conducted as a laboratory work in which the code of professional ethics regarding laboratory animals was entirely complied with. The animals tested here include 56 adult male Wistar rats with the average weight of 200 - 220 g, aged 2.5 - 3 months old were brought from the center for animal breeding and maintenance at Shiraz University. The rats were divided into seven groups of eight animals. During the whole 28 days of the experiment, they were exposed to 12: 12 hours light-dark cycle. During the whole test, animals were fed with compressed food products supplied by Shiraz Livestock and Poultry Co. During the test, the temperature was 25°C and light was emitting indirectly through the windows of the laboratory. The rats were divided into seven groups of eight animals: Group 1 [as the control group]: The members of this group did not receive any pharmaceutical solvents. Group 2 [as the sham group]: The members of this group were injected 0.2 mL distilled water intraperitoneally as a pharmaceutical solvent every day. Group 3 [as experimental group I]: The members of this group were received 5 mg/kg/day cyclophosphamide i.p. every day and 200 mg/kg/day hydro alcoholic extract of garlic orally. Group 4 [as experimental group II]: The members of this group were received 5 mg/kg/day cyclophosphamide i.p. every day and 400 mg/kg/day hydro alcoholic extract of garlic orally. Group 5 [as experimental group III]: The members of this group were received 5 mg/kg/day cyclophosphamide i.p. every day and 800 mg/kg/day hydro alcoholic extract of garlic orally. Group 6 [as experimental group IV]: The members of this group were received 5 mg/kg/day cyclophosphamide i.p. every day. Group 7 [as experimental group V]: The members of this group were fed 800 mg/kg/day hydro alcoholic extract of garlic.
The animals received oral administration of garlic extract and cyclophosphamide i.p. for 28 days as per Table 1.
| Parameter | Control | Sham | I | II | III | IV | V |
|---|---|---|---|---|---|---|---|
| 5.13 ± 0.28 | 5.18 ± 0.390 | 4.01 ± 0.246 | 0.161 ± 4.83 | 5.02 ± 0.278 | 3.93 ± 0.691 | 6.11 ± 0.230 | |
| 8.97 ± 0.615 | 8.52 ± 0.366 | 9.01 ± 0.249 | 9.12 ± 0.53 | 9.99 ± 0.333 c | 8.07 ± 0.607 | 10.62 ± 0.532 | |
| 0.050 ± 1.42 | 1.40 ± 0.069 | 1.24 ± 0.04 | 1.3 ± 0.03 | 1.34 ± 0.015 | 1.18 ± 0.030 | 1.43 ± 0.040 |
After conducting the experiment and treating for 28 days, the animals were anesthetized by ether in anesthesia containers (made by Kimia Mavad Company, Iran). After opening chests of rats, blood samples were taken from their ventricular area by 5 mL syringes. The blood samples were transferred to the special tubes and their serum samples were obtained using a centrifuge unit (made in Germany) with 3000 RPM for 5 min. The serum samples were transferred to some separate tubes using a Pasteur pipette (Kimia Material Company, Iran) and stored at 20°C until hormone measurement. The amount of LH, FSH hormones and testosterone were determined using the Radioimmunoassay (RIA) method [using specific rat kits (Specteria, Finland)] using a gamma counter unit (made in Germany) in terms of mLU/mL.
The results were studied based on SPSS-16 statistical program, Tukey tests, and ANOVA and the significant difference of P ≥ 0.05 were considered. In this method, the unprocessed data were imported by SPSS-16 software, Package for the Social Sciences (SPSS Inc, Chicago, IL). Tukey tests and ANOVA were used for studying and comparing the results obtained from the groups. The relevant histograms were drawn using excel based on the obtained data and the data were analyzed.
4. Results
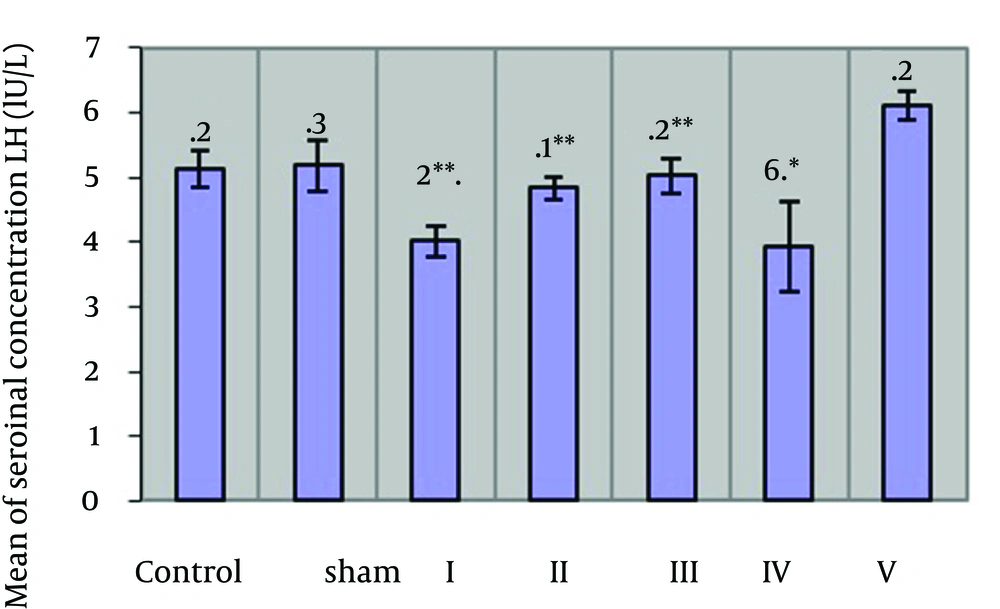
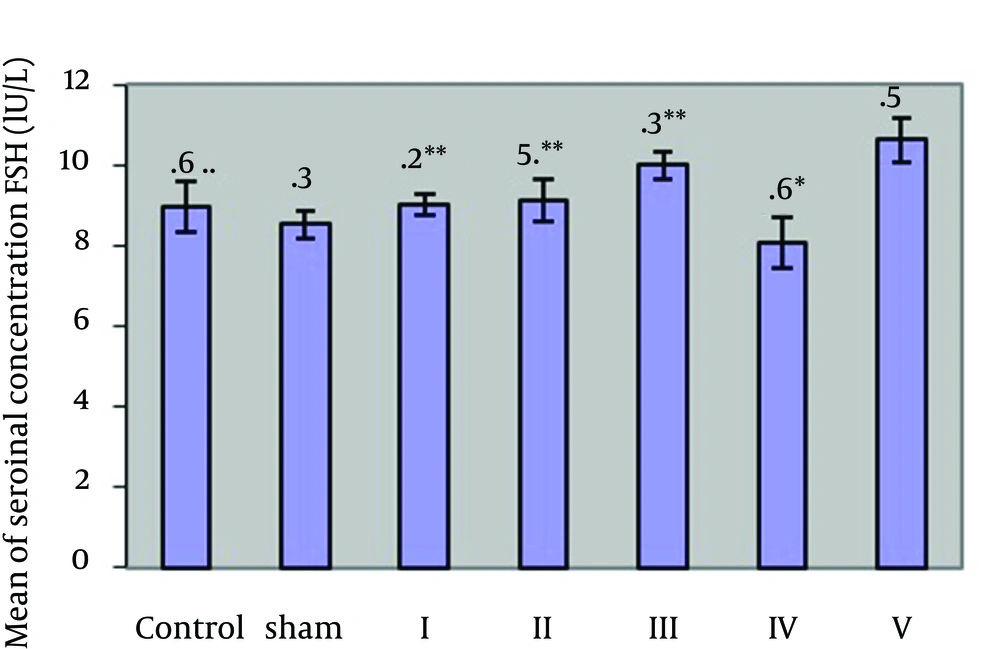
Table 1 and Figure 1 show changes of serum concentration of LH hormone in the different groups. According to the Table 1, mean of serum concentrations of LH hormone in the group 4 shows a significant reduction at (P ≥ 0.05) level as compared with the case and control group. The groups I - III showed a significant increase at (P ≥ 0.05) level as compared with the group 4. Table 1 and Figure 2 show changes of serum concentration of FSH hormone in the different groups. As the Table 1 shows, mean of serum concentration of FSH hormone in the groups I - V show a significant increase at (P ≥ 0.05) level as compared with the case groups. There is a significant increase at (P ≥ 0.05) level between the groups I, II, III and the group IV.
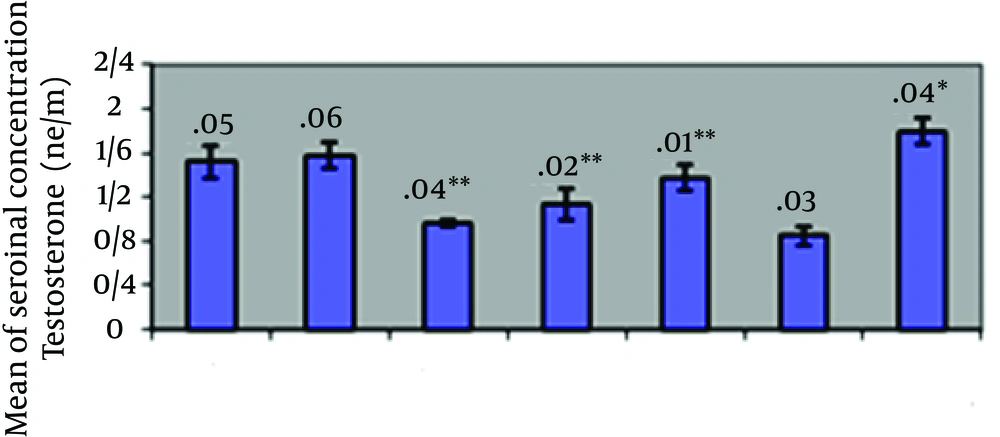
Table 1 and Figure 3 show changes of serum concentration of testosterone hormone in the different groups. As the Table 1 shows, mean of serum concentration of testosterone hormone in the group 4 shows a significant reduction at (P ≥ 0.05) level as compared with the case group. There is a significant increase at (P ≥ 0.05) level as compared with the group IV.
5. Discussion
The results of this study showed that compounds in the garlic controls the side destructive effects of cyclophosphamide and its metabolites. Cyclophosphamide (Endoxan) is an anticancer drug. In body, this anti neoplastic drug (anticancer drug) is changed into an alkylating active metabolite or similar effects of mustine. Factors such as changes of concentration of LH and FSH hormones and testosterone were studied. The studies show that LH, FSH and testosterone have a fundamental role in spermatogenesis [7]. According to the mentioned effect on phenolic glycoside in rhizome and its antioxidant properties, which are effective in spermatogenesis [8, 9] and since such compound is in garlic, it can be stated that this compound is one of the active and effective compounds to increase spermatogenesis [9, 10]. Therefore, it can be concluded that the increase of spermatogenesis, due to garlic extract consumption, indicates the increase of weight of testes and finally the increased secretion of sex hormones. Garlic presumably strengthens its antioxidant action through increasing removal of free radicals, activity of antioxidant activity of superoxide dismutase, glutathione peroxide, and increasing glutathione level [11]. Antioxidant ability of garlic depends on the presence of organic sulfur compounds that regulate glutathione level and GST activity [12]. Therefore, they are able to remove the harmful effects of cyclophosphamide and its active metabolite so that the higher the dose of garlic extract is, the less such harmful effects are seen. Garlic extract protects DNA from the damages caused by free radicals and cyclophosphamide metabolites. In case the DNA of the testes cells is damaged due to cyclophosphamide metabolites, the garlic compounds recover them [11, 13, 14]. Using the results of Table 1 and Figure 1, we can conclude that by consumption of cyclophosphamide in the experimental group IV, LH hormone shows a significant reduction as compared with the control group. This is due to the effect of active metabolites such as acrolein caused by metabolism of cyclophosphamide in body. By affecting DNA molecule, breaking it, affecting RNA molecules, and synthesizing proteins, cyclophosphamide may cause many side effects [1, 2]. If they affect the LH generating cells located in the anterior pituitary, serum level of LH hormone becomes lower. If they affect hypothalamus and GnRH-generating cells, number of GnRH produced hormone decreases considerably and GnRH reduction, in turn, leads to lowering LH hormone. After DNA molecule breakage, replication does not occur, which is followed by protein synthesis reduction. Therefore, LH and GnRH levels decrease with respect to their chemical structure, which is protein (polypeptide) [15].
By increasing dose of garlic extract, LH hormone content of the members of the groups I, II, III who received cyclophosphamide, increased as compared with those who only received cyclophosphamide. In the groups II and III, it shows a significant increase as compared with the experimental group IV. The increase of LH hormone in the group members who received garlic extract can be attributed to its compounds. Some of the compounds of garlic, especially sulfur compounds, are activated by oxidation of lipids and tissue atrophy [10, 16]. Therefore, garlic compounds lead to removal of active metabolites of cyclophosphamide (acrolein). With the dose of garlic extract increasing in the groups I, II, III, the probability of removal and prevention of active metabolites increases and LH hormone concentration increases as compared to the group IV. With the garlic extract increasing, the harmful effects of the drug decreases. This increase is significant in the groups II and III as compared to the group IV. The studies show that the increase of free radicals leaves adverse effects on proliferation, activity, and fertilization of sperms. In case level of these radicals is not adjusted regularly, it hinders natural performance of cells. According to the studies, the complex process of spermatogenesis and passing through germinal to reach maturity level of sex cells depend on being protected from the pathologic and cytotoxic damages that threaten this phenomenon [17, 18]. Using the results of Table 1 and Figure 2, we can conclude that consumption of cyclophosphamide in the group IV leads to a significant decrease in FSH as compared to the case group. This reduction may be due to the effect of cyclophosphamide on DNA, its breakage, and the effect of RNA and reduction of protein synthesis [1, 2]. As FSH hormone has a polypeptide structure, the drug may reduce FSH level in the group IV. Some active metabolites of cyclophosphamide, such as (acrolein), which has many harmful effects on genitourinary tract, may reduce FSH level. FSH hormone level in the group V shows a significant increase as compared with the case group. FSH hormone in the groups I, II and III has a significant increase as compared with the group IV. Therefore, the antioxidant compounds in garlic (Sulfur-containing compounds that are abundant in garlic) by removing active and negative metabolites from body, reduce the effect of such metabolites on the anterior pituitary secreting FSH hormone [19].
Using the results of Table 1 and Figure 3, we can conclude that consumption of cyclophosphamide in the group IV led to reduction of testosterone hormone, which is significant as compared with the case group. As per the earlier studies [20, 21] reduction of concentration of testosterone hormone due to consumption of cyclophosphamide can be proved and it has already been proved. One of the reasons of reducing concentration of testosterone hormone caused by the effect of active metabolites of cyclophosphamide on DNA and DNA molecule breakage in cells [1, 2, 20] one of the cells may be interstitial cell (Leydig). The catalyzing enzymes of testosterone hormone were not produced in these cells, which led to lowering concentration of testosterone hormone. Using the results of Table 1 and Figure 1, we can conclude that cyclophosphamide reduces LH hormone concentration and LH directly affects Leydig cells and leads to secretion of testosterone hormone. Decrease of LH hormone concentration leads to lowering concentrations of testosterone hormone.
In the groups I, II, III, the more the garlic extracts increases, the more the testosterone hormone increases with respect to the group IV, as the increase in the groups II and III is significant as compared with the group IV. Moreover, in the group V, who only received the extract, a significant increase was seen as compared with the case and control groups; garlic extract compounds are responsible for the significance increase. By affecting the performance of steroid-generating enzymes, the effective compounds of garlic extract increase secretion of testosterone hormone and its metabolites. FSH hormone increases synthesis and secretion of ABP and provides concentration of testosterone inside the seminiferous tubules for the normal process of spermatogenesis [22]. Therefore, according to Table 1 and Figure 2, the significant increase of FSH hormone in the group II and III as compared with the group IV and the increase of testosterone hormone in these groups as compared with the group 4 are expected. It was proved that calcium might be an effective molecule for expressing cooperation between FSH and activity of the testosterone on Leydig cells, which strengthens sperm-generating [23]. As calcium is one of the compounds of garlic [24], such process is not beyond expectation. The studies show that antioxidants lead to controlling superoxide and hydroxyl radicals and prevent concretization of cells and tissues, including testes tissue considerably [25, 26]. Therefore, it can be concluded that antioxidant compounds in garlic prevent disorders of cells and tissues activity, especially testicular tissues responsible for secretion of hormones and improve the damages caused by consuming chemical drugs. Antioxidant compounds, especially sulfur-containing compounds in garlic extract, eliminate free radicals and the active metabolites created in body. Moreover, garlic increases activity of antioxidant enzymes of superoxide, dismutase, peroxidase, glutathione peroxide [11, 27, 28]. Therefore, garlic compounds reduce the harmful effects of this drug on reproductive system and secretion of sex hormones. With the dose of garlic extract increasing, such negative effects decrease. The results of this research showed that prescribing hydro alcoholic extract of garlic during 28 days of experimental could partially increase serum concentration of LH and FSH hormones and testosterone obtained from consumption of cyclophosphamide. Meanwhile, with respect to the time restriction of the present research, the long-term effects of hydro alcoholic should be evaluated. In case the results are consistent, including this plant in the diet of those whose reproduction activity is distorted due to consuming chemical drugs may help them to remove such disorder.