1. Background
Gelatin is a soluble protein obtained by partial hydrolysis of collagen, the main insoluble fibrous protein constituent on bones, cartilages and skins [1]. Unique collagen and gelatin structures influence their physical properties, such as solubility, swelling, water uptake, moisture absorption, water evaporation, transparency, color, odor, gel strength and thermal stability. Physical properties of gelatin itself influence gelatin quality and potential applications in food and pharmaceutical industry [2, 3]. The food and pharmaceutical applications of gelatin are based mainly on the gel-forming, film-forming and viscoelastic properties. Recently, an increasing number of new applications have been found for gelatin in products, such as emulsifiers, foaming agents, colloid stabilizer, hydrogels, fining agents, packaging materials, wound dressing and micro-encapsulating agents [4, 5]. Gelatin has been reported to be one of the first materials used as carrier of bioactive components. Enrichment of nano-capsules with natural antioxidants and/or anti-microbial functional properties of these nano-capsules will increase. There is growing interest in using plant extracts as natural sources of antioxidant/antibacterial compounds in the formulation of gelatin nano-capsules [6-9]. In this context, plant essential oils and their main components are gaining a wide interest in health industry for their potentials as antioxidant and antimicrobial agents, as they are generally recognized as safe (GRAS) [10, 11]. To our knowledge there is no report on the on the antioxidant and antimicrobial activity of gelatin films incorporated with Ferula assa-foetida. F. assa-foetida is a useful plant in the Apiaceous family. In traditional medicine the plant is used for the treatment of different diseases, such as asthma, epilepsy, stomachache, flatulence, intestinal parasites, weak digestion and influenza [12, 13]. In this study the gelatin nano-capsules with antioxidant and antimicrobial activities were prepared from gelatin solutions containing different F. assa-foetida essential oil concentrations. Antioxidant activities of the gelatin nano-capsules incorporated with F. assa-foetida essential oil were examined using 2′-azino-di (3 - ethylbenzthiazoline -6-sulfonate) (ABTS) decolorization. The gelatin nano-capsules containing F. assa-foetida essential oil were individually tested against two Gram-negative bacteria (Pseudomonas aeroginosa and Escherichia coli) and two Gram-positive bacteria (Staphylococcus aureus and Bacillus subtilis) commonly found in human pathogenesis.
2. Objectives
In this study, we proved that gelatin nano-capsules grafted with F. assa-foetida essential oil have excellent physical, well as good antioxidant and antibacterial activity, which is suitable for producing nano-capsules of antioxidant antibacterial drug.
3. Materials and Methods
3.1. Preparation of Gelatin Nano-Capsules Forming Solutions
In this experimental study, to prepare gelatin nano-capsules forming solutions, 10 g of bovine gelatin powder (Sigma-Aldreich, Germany) dissolved into 80 mL of distilled water at ambient temperature and temperature increased to 60°C using a Hotplate-stirrer and the mixture was stirred for 30 minutes at this temperature. After cooling to 37°C, F. assa-foetida essential oil (obtained by hydro-distillation) with different concentration (2, 4, 6, and 8% w/w based on the weight of the gelatin powder = 1, 2, 4, and 8 mg/mL based on the gelatin solutions) as antimicrobial agent, glycerol (25% w/w based on the weight of the gelatin powder = 25 mg/mL based on the gelatin solution) (Merck, Germany) as plasticizer and glutaraldehyde (2% w/w based on the weight of the gelatin powder = 2 mg/mL based on the gelatin solution) (Merck, Germany) as cross-linker were added to gelatin solutions and then distilled water was added to final of 100 mL and homogenized [14, 15].
3.2. Preparation of Gelatin Nano-Capsules
To cast the films, 10 mL of gelatin film forming solutions containing different F. assa-foetida essential oil concentrations were transferred into the polyester Petri dish (Farazbin Kimia Co., Teheran, Iran, the radius of 74 mm) and placed at room temperature until films were dried. Then grind the dried film to be produced gelatin nano-capsules.Ferula asa-foetida essential oil reserves are in the nano-capsules.
3.3. Surface Morphology and Nano-Capsules Microstructure
The test was conducted at the Department of Materials Engineering, Shiraz University. Silver particles coated with gelatin nano-capsules of F. assa-foetida essential oil.
Antioxidant activity of nano-capsules: Antioxidant activities of the films were determined by decolorization method with 2, 2′-azino-di (3-ethylbenzthiazoline-6-sulfonate) (ABTS, Sigma, Germany) [16]. The method was modified to detect the continuous antioxidant release from films. The release tests were performed in 24 well plates. Briefly, 20 mg powder nano-capsules from different parts of the nano-capsules containing 2, 4, 6 and 8% of the F. assa-foetida essential oil was added to 2.0 mL of diluted ABTS radical solution (7 mM ABTS and 2.54 mM potassium persulfate, A734 = 1 ± 0.1). Nano-capsules without F. assa-foetida essential oil were used as blank. The program was adjusted to record the absorbance values after shaking the 24 well plates for 30 seconds using a plate reader (Bio Tek Elx 808, Winooski, VT, 05403, USA). The data was recorded up to steady state was reached for each sample. A standard curve of ascorbic acid ranging from 0.44 to 15.76 mg/mL was prepared. Antioxidant activity was expressed as mg ascorbic acid equivalents per gram of films using standard curve.
3.4. Antibacterial Activity of Gelatin Nano-Capsules Using Colony Counting
All microorganisms obtained from the Persian type culture collection (PTCC), Tehran, Iran. The powder nano-capsules were individually tested against two Gram-negative bacteria [P. aeruginosa PTCC 1074 (ATCC 9027 and E. coli PTCC 1330 (ATCC 8739)] and two Gram-positive bacteria [S. aureus PTCC 1112 (ATCC 6538) and B. subtilis PTCC 1023 (ATCC 6633)]. The bacterial colony counting assays were carried according to Clinical and Laboratory Standards Institute (CLSI) and ASTM G22-76. Bacteria strains were suspended in LB media and the densities were adjusted to 0.5 McFarland standards at 640 nm (108 CFU/mL) and then diluted to 105 CFU/mL with LB. Twenty milligram powder nano-capsules in a 10 mL liquid culture containing 10 µL microbe cultures. Then, the sample was incubated at 37°C for 24 hours (Shaking Incubator, Shin Saeng, Fine Tech, Korea). From the incubated samples, a 100 µL solution was taken and diluted with the appropriate dilution factor and the final diluted microbe solution was plated and distributed onto nutrient agar plate (Farazbin Kimia Co., Tehran, Iran). The plates cultured with the powder nano-capsules without F. assa-foetida essential oil under the same condition were used as control. All plates were incubated at 37°C for 24 hours and the numbers of colonies that formed were counted. The antibacterial efficacy of the powder nano-capsules was calculated according to the following equation [17]. Colony reduction (%) = [(Number of colony in test samples - Number of colony in control)/ Number of colony in test samples] × 100.
3.5. Statistical Analysis
Data are expressed as the means plus/minus standard deviations of at least three independent experiments. The significant differences between treatments were analyzed by one-way analysis of variance (ANOVA) and Duncan tests at P < 0.05 using statistical package for the social sciences (SPSS-17, Abaus Concepts, Berkeley, CA) and Prism 5 (Graph Pad, San Diego, USA) software’s.
4. Results
4.1. Plant Materials
GC-MS analysis of the F. assa-foetida essential oil indicated the main components were β-pinene (47.1%), α-pinene (21.36%), 1, 2-dithiolane (18.6%), nitrite propyl (3.67%), thionol (2.64%) and cis-β-ocimene (2.43%).
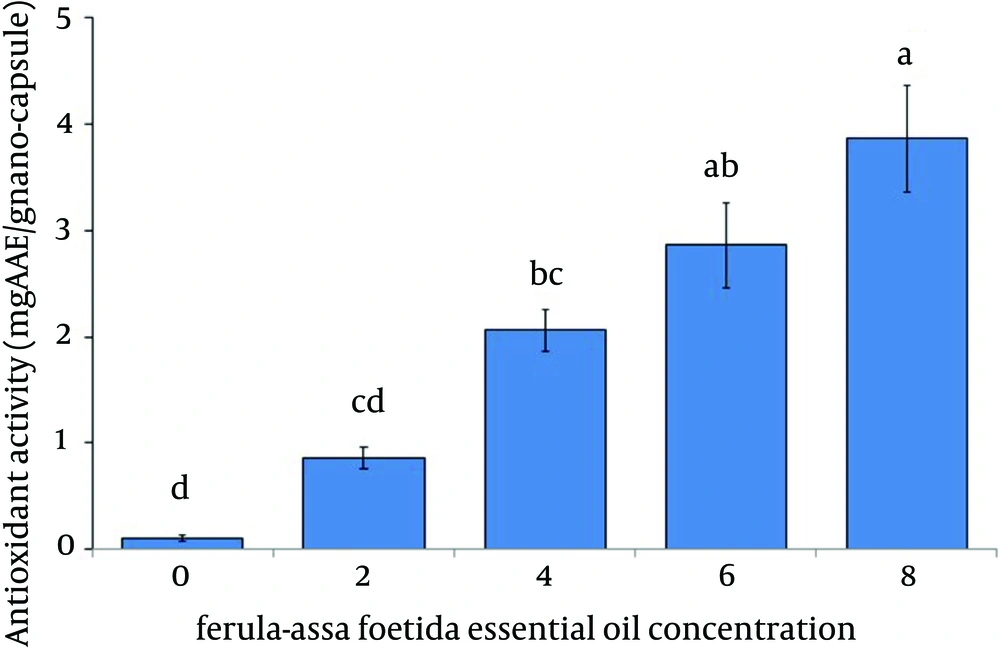
Antioxidant activity of nano-capsules: Antioxidant activity of the gelatin nano-capsules incorporated with different F. assa-foetida essential oil concentrations was determined by ABTS decolorization method and expressed as mg ascorbic acid equivalent per gram of nano-capsules (Figure 1). The gelatin nano-capsules without F. assa-foetida essential oil showed very low activity against the ABTS decolorization. The antioxidant activity was expressed milligram ascorbic acid equivalent (AAE) per gram incorporating different concentrations of F. assa-foetida essential oil. Mean values with different letters within a column are significantly different by Duncan’s multiple range tests at (P < 0.05).
4.2. Morphology of Nano-Capsules
SEM analyses of the results are shown in Figures 2 - 4. The SEM images at magnifications of (100 ×) gelatin nano-capsules containing different F. assa extract of bitter are more compact than 4% nano-gelatin concentrations (0, 4 and 8) of F. assa-foetida of bitter. Gelatin nano-capsules with 8% of Ferula capsules gelatin films of F. assa-foetida bitter are very less squeezed. Due to the hydrophobic nature of F. assa-foetida they bonds with second hydrophobic gelatins which this causes crosslinking of the film is broken and links between Ferula assa-foetida and second’s gelatin is more intense and gelatin nano-capsules containing a larger percentage of the F. assa-foetida of bitter are more compact F. assa-foetida of bitter towards nano-capsules without the additional bonds with the hydrophobic domains formed gelatin. As a result, increases the concentration of F. assa-foetida carrier oil gelatin capsules are more compact and can store larger percentage of F. assa-foetida extract.
4.3. Antibacterial Activity of Gelatin Nano-Capsules
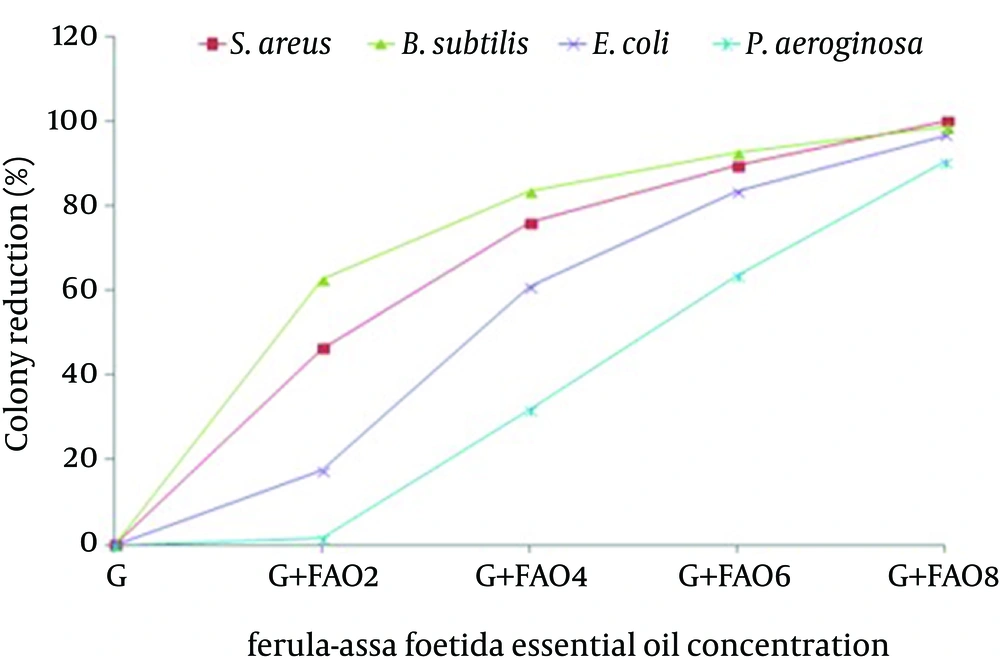
Thus gelatin films incorporated with F. assa-foetida essential oil are effective against both Gram-positive and Gram-negative bacteria while they are more effective to Gram-positive bacteria rather than to Gram-negative bacteria. The results of colony reduction percentage are summarized in (Figure 5).
According to the results obtained, the antibacterial activity of gelatin nano-capsules containing different F. assa essential oil concentrations was greatest against S. aureus followed by B. subtilis followed by E. colli and then by p. aeroginosa F. assa-foetida essential oil gradually released from films to the solution and penetrates to the cell membranes and disrupts membrane structure and finally cell death. Antibacterial activity was expressed as bacterial growth reduction in the presence of nano-capsules with different Ferula oil concentration.
Mean values with different letters within a column are significantly different by Duncan’s multiple range tests at (P < 0.05).
5. Discussion
According to the results, gelatin nano-capsules are good nominations for a variety of antibiotic replacement industrial. Previous study reported the main components of the F. assa-foetida essential oil, were (E)-1-propenyl-sec-butyl disulfide (40%), (Z)-1-propenyl-sec-butyl disulfide (8.7%), germacrene B (7.8%), α-pinene (5.9%) and β-pinene (5%) [15]. Thus, the F. assa-foetida essential oil analyzed in this research had roughly same components with other previously analyzed F. assa-foetida essential oil, however, were showed important differences in their quality and quantity of the components. Various studies have examined antioxidant properties of peptides derived from gelatin in different sources. These studies have shown peptides derived from enzymatic hydrolysis of gelatin are lipid peroxidation inhibitors, free radical scavengers, and transition metal ion chelators. The antioxidative properties of peptides are related to their amino acid composition, molecular weight structure, and hydrophobicity [18, 19].
The gelatin nano-capsules containing different F. assa-foetida essential oil concentrations decolorize ABTS dose-dependently. Fish skin gelatin film incorporated with citrus essential oils [16] were exhibited strong antioxidant activity, which similar to our results. These antioxidant activity may be attributed, at least in part, to the presence of phenols, flavonoids, sesquiterpenes and sulfur-containing compounds. These studies have shown peptides derived from enzymatic hydrolysis of gelatin are lipid peroxidation inhibitors, free radical scavengers and transition metal ion chelators. The antioxidative properties of peptides are related to their amino acid composition, molecular weight, structure and hydrophobicity [4, 19].
The gelatin nano-capsules containing different F. assa-foetida essential oil concentrations decolorize ABTS dose-dependently. Fish skin gelatin film incorporated with citrus essential oils [16] were exhibited strong antioxidant activity, which similar to our results. This antioxidant activity may be attributed, at least in part, to the presence of phenols, flavonoids, sesquiterpenes and sulfur-containing compounds in the F. assa-foetida essential oil [18, 20]. These results recommended that gelatin nano-capsules incorporated with F. assa-foetida essential oil could be promising candidate for safe radical scavenger in the body. Due to the hydrophobic nature of F. assa-foetida they bonds with second hydrophobic gelatins which this causes crosslinking of the film is broken and links between F. assa-foetida and second’s gelatin is more intense and gelatin nano-capsules containing a larger percentage of the F. assa-foetida of bitter are more compact F. assa-foetida of bitter towards nano-capsules without the additional bonds with the hydrophobic domains formed gelatin. As a result, increases the concentration of F. assa-foetida carrier oil gelatin capsules are more compact and can store larger percentage of F. assa-foetida extract. Gelatin film from the skin of unicorn leatherjacket incorporated with essential oils [16] displayed excellent antibacterial activities, which similar to our results. The antibacterial activity which recognized in the essential oils from several medicinal plants established that the essential oils is related with the attack on the phospholipids present in the cell membranes, which causes increased permeability and leakage of cytoplasm, or in their interaction with enzymes located on the cell wall. Thus, the resistance of Gram-negative bacteria to the essential oils likely lay in the protective role of their cell wall lipopolysaccharide or outer membranes proteins, which restricts diffusion of hydrophobic compounds through its lipopolysaccharide layer [21].
Essential oils have the ability to disrupt lipid structure of the cell wall of bacteria, leading to destruction of cell membrane, cytoplasmic leakage, cell lysis and ultimately cell death. The decrease in pH that occurs due to cell membrane disruption resulted in a loss of control of cellular process such as ATP biosynthesis, DNA transcription and protein synthesis [22]. F. assa-foetida essential oil gradually released from films to the solution and penetrates to the cell membranes and disrupts membrane structure and finally cell death. The nano-capsules as well as due to the risk of bacterial resistance stay safe. The nano-capsules according to the origin of the whole plant does not have any negative effects on the body.