1. Background
Diabetes mellitus is a metabolic disorder which can have destructive effects on the central nervous system. Although perfect mechanisms for mediating the effects of diabetes on the brain have not been identified yet, it has been shown that several factors such as oxidative stress, neuroinflammation mitochondrial dysfunction, neuropathic apoptosis, impaired insulin signaling, and neurogenesis are involved in the damage to the central nervous system during diabetes (1).
In diabetes, the metabolism of proteins, lipids, and carbohydrates changes that lead to the increase in oxidative stress and neural cell death in different parts of brain. Moreover, hyperglycemia caused by diabetes weakens the coordination of the motor system, and consequently decreases motor activity (2).
The cerebellum plays a key role in controlling and implementing several aspects of the motor function, such as coordination, muscle tone, and motion (3). The cerebellum, as a part of the central nervous system, has a close relationship with such important factors as emotion, cognition, and behavior. Besides, any damage and change to the cerebellum can cause motor weakness, dementia, schizophrenia, and other mental disorders (4). Type 1 diabetes can cause a disorder in the cerebellar structure and hypoplasia of the cerebellum. Moreover, hyperglycemia decreases the number of cells and the thickness of gray and white matters of the cerebellum (5, 6). However, few studies have been conducted on cellular changes in the cerebellum following diabetes, and the protective effects of diabetes on the cerebellum have not been fully elucidated yet (7).
Diabetes control using chemical drugs may have some drawbacks; thus, achieving some effective, low-cost, natural, and safe compounds to control the disease is necessary. Traditional diabetes treatments such as herbal extracts are known worldwide (8). In this regard, the hypoglycemic effects of Aloe vera gel have been reported frequently (9). Aloe vera has many applications in cosmetic and medical products since it is a rich source of diverse phytochemical products like polysaccharides, flavonoids, tannins, glycoproteins, enzymes, alkaloids, anthraquinones, intranas, coumarins, chromones, vitamins, minerals, carbohydrates, and proteins (10). Many biological effects of A. vera, including neuroprotective, anti-inflammatory (11, 12), antioxidant (13), as well as anti-diabetic effects, have been reported in both experimental and clinical studies (14). Toxicological studies demonstrated that sub-chronic and chronic administration of A. vera in rats and mice has no toxic effects (15-17).
Extensive effects of A. vera gel on diabetes have been investigated in clinical and experimental studies. However, as far as the researchers of this study investigated, no study has examined the effect of A. vera on histomorphometrical changes in the cerebellum of diabetic animals so far.
2. Objectives
The present study aimed to evaluate the protective effect of A. vera gel on histomorphometrical changes in cerebellum of streptozotocin (STZ)-induced diabetic rats.
3. Methods
3.1. Chemicals and Drugs
In this study, we used the following chemicals and drugs: (1) A. vera gel (Barij Essence Pharmaceutical Co., Kashan, Iran); (2) human NPH insulin (Lansolin® N, Exir Pharmaceutical Co., Boroujerd, Iran); (3) streptozotocin (STZ; Enzo, Life sciences, Inc. USA); (4) sodium citrate; (5) citric acid (Sigma-Aldrich Co., England, UK); (6) ketamine and Xylazine (Alfasan Chemical Co., Netherlands).
3.2. Animals
A total of 25 male Wistar rats (weighting 200 - 210 g and 12 weeks old) were prepared from the central animal house of Jundishapur University of Medical Sciences, Ahvaz, Iran. The animals were kept at 22°C and 12: 12h light/dark. During the experiment, feeding was done with standard pellets and urban water pipelines. The applied experimental protocols were confirmed by the Ethics Committee of Shahid Chamran University of Ahvaz (approval ref no.EE/95.12.4/9025601/scu.ac.ir) and used by the International Animal Testing Guidelines.
After one week adaptation, diabetes was induced using 60 mg/kg STZ dissolved in citrate buffer (0.1 m, pH = 4.5) intraperitoneally (IP) (18). The same volume to other rats (2 mL/kg) was injected from the citrate buffer. The induction of experimental diabetes was evaluated three days after STZ injection. One week after STZ injection, blood glucose level was assessed by a glucometer through the tail prick. Only animals with blood glucose levels over 250 mg/dL were accepted as diabetic rats. The onset of Disease was considered the day on which hyperglycemia was confirmed. Blood glucose and body weight measurements were taken at the beginning and end of the study.
After diabetes confirmation, both healthy and diabetic animals were randomly assigned into five groups as follows:
- Control group: The rats received a daily physiological serum equivalent to the volume of condensed commercial A. vera gel (1 mL/kg/; gavage).
- Aloe vera group: The healthy rats fed with 8 mL/kg of condensed commercial A. vera gel daily.
- STZ group: Animals in this group became diabetic (60 mg/kg STZ dissolved in citrate buffer (0.1 m, pH = 4.5) by IP and received the same daily physiological serum as the first group.
- STZ + A. vera group: The diabetic rats treated with daily A. vera gel (400 mg/kg/gavage).
- STZ + insulin group: Diabetic rats treated by human NPH insulin (10 IU/kg daily, subcutaneous (SC) (19).
The experiment continued for eight weeks.
3.3. Sampling
The rat brains were removed intact immediately after being anesthetized with a ketamine-xylazine mixture (100 mg/kg - 10 mg/kg, respectively). In the next step, the cerebellum was removed from the brain. Then, the samples were washed with physiological serum and transferred to a 10% buffered formalin solution for fixation.
3.4. Histomorphometrical Studies
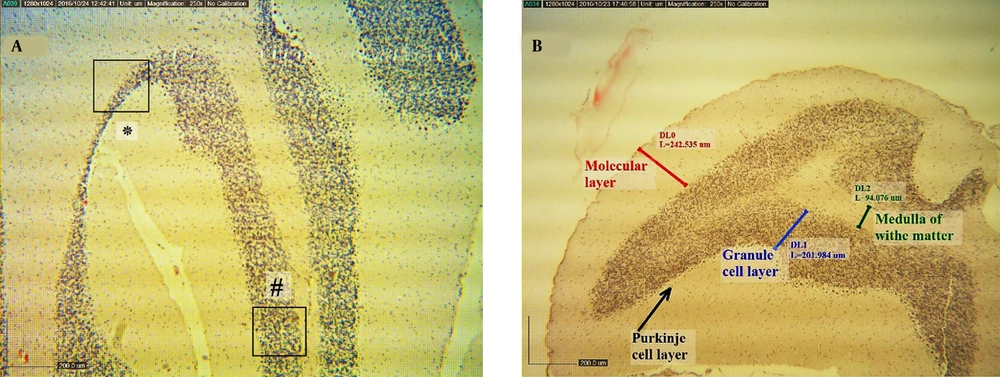
The 5 - 6 µm tissue sections were made using paraffin embedding method and stained hematoxylin-eosin (H & E). In microscopic studies, the number of purkinje cells in 100 μm length, the number of glial cells, and the number of granular cells in 5000 µm2 in the granular layer of the animal cerebellum were counted. Also, the thickness of molecular, purkinje, granular, and white matter layers at the apex and depth of cerebellum lobules in all groups were evaluated (Figure 1). The cell counting per unit area was done by the Japanese Olympus microscope and Dino-capture II software. In this assessment, at least five regions of each of the three layers of the cerebellum were examined in each slide, and their mean values were recorded separately.
A, a representative coronal section of a simple sulcus of cerebellum in a normal rat (H & E stain, scale bar 200 µm) (#illustrates the apex of lobule in cube; *represents depth of sulcus). B, shows thickness of molecular, purkinje, granular, and white matter layers at the apex of lobule and depth of cerebellum sulcus (H & E stain, scale bar 20 μm).
3.5. Statistical Analysis
The SPSS software version 24 (SPSS Inc., Chicago, IL) was used for statistical analysis. The results were expressed as mean ± standard error of the mean (mean ± SEM), and the treatments were compared using one-way analysis of variance (ANOVA). In all cases, P < 0.001 was considered as a statistically significant difference.
4. Results
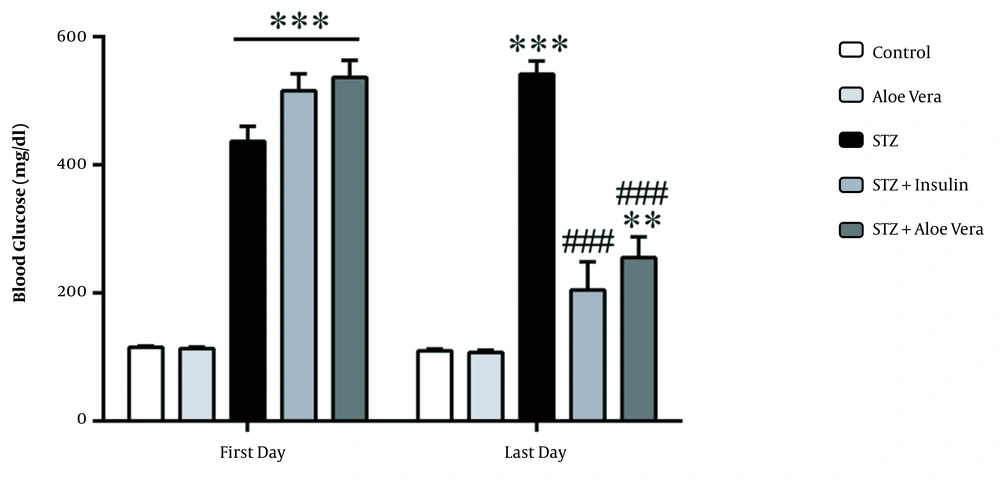
As shown in Figure 2, blood glucose levels in different groups changed significantly (P < 0.001). The blood glucose measurements in diabetic rats after 8-week treatment period showed significant variations among different groups (P < 0.001). The blood glucose levels in diabetic rats treated by A. vera and insulin were reduced when compared with the diabetic group (P < 0.001). No significant differences in blood glucose levels were observed among insulin-treated group and the group treated with A. vera gel.
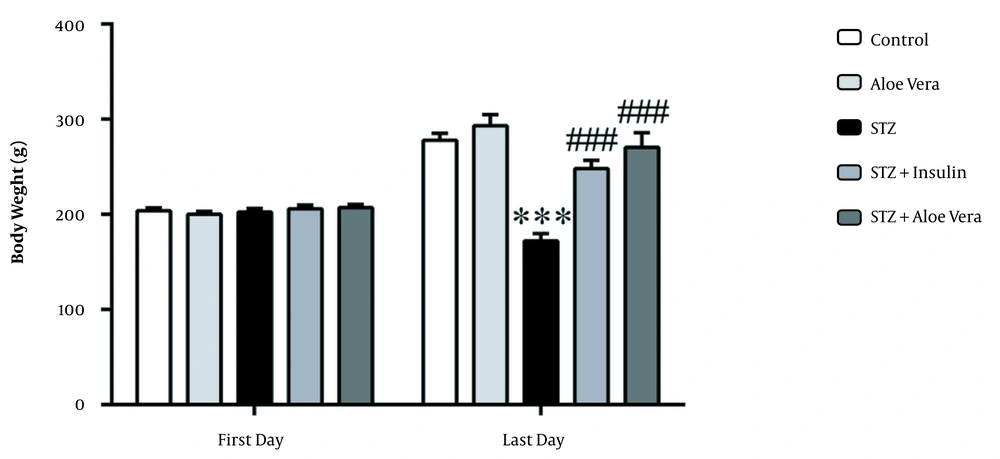
As shown in Figure 3, the mean body weight of diabetic animals reduced from 202.6 ± 3.6 to 172 ± 7.9 g after eight weeks. However, the body weight of the normal animals enhanced from 203.9 ± 3 to 277.7 ± 7.6 g; at the end of the eighth week, the difference in body weight between the two groups was significant (P < 0.001). Mean weight of the untreated diabetic rats at the end of the eighth week was markedly lower than the A. vera gel and insulin groups (P < 0.001). Also, daily treatment of diabetic rats with A. vera gel and insulin prevented their weight loss. Accordingly, the body weight of healthy rats receiving A. vera enhanced, although it was not statistically significant (P > 0.05).
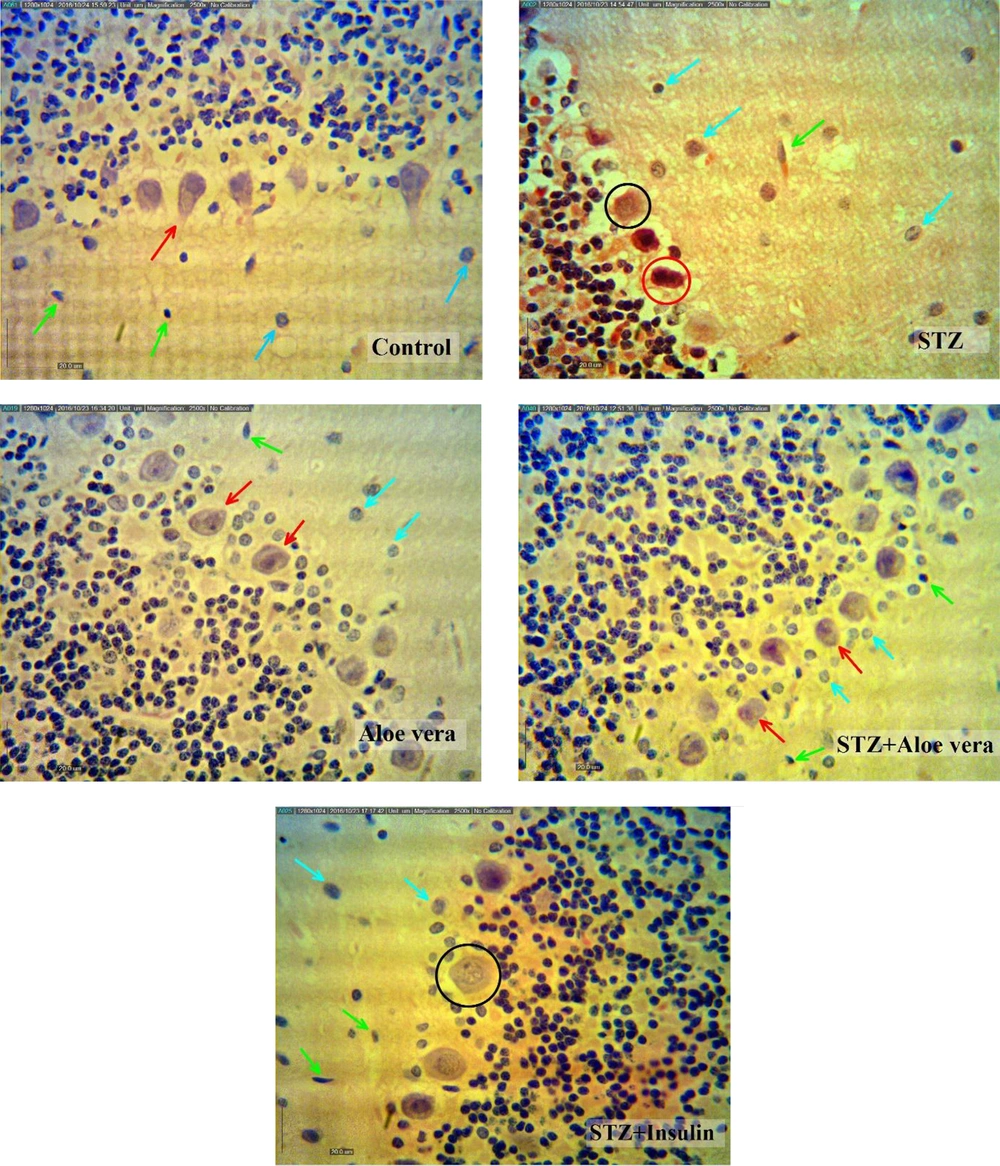
The results of histomorphometrical studies (Figures 1 and 4; Table 1) showed that the number of granular and purkinje cells of the cerebellum in the STZ group significantly decreased (P < 0.001) compared with the control group, while the treatment of animals with insulin or A. vera improved these changes (P < 0.001). Moreover, the number of glial cells in the cerebellum molecular section in the STZ group showed a significant increase (P < 0.001) compared to the control group. Treatment of diabetic animals with insulin or A. vera improved this change and returned to the control level (P < 0.001).
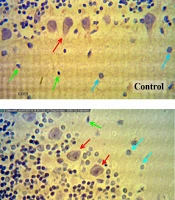
Cerebellum structure changes in different groups (H&E stain, scale bar 20 µm) Purkinje cells with dendrites and central cores (red arrow), astrocytes (blue arrow), microglia (green arrow), the purkinje cells of the piconotic (red circle), a very small number of purkinje cells are also vacuated (black circle).
| Groups | Number of Purkinje cells (100 µm) | Number of Glial Cells (5000 µm)2 | The Number of Granular Cells (5000 µm)2 |
|---|---|---|---|
| Control | 5.80 ± 0.37 | 4.80 ± 0.37 | 147.40 ± 4.38 |
| Aloe vera | 5.60 ± 0.40 | 5.60 ± 0.60 | 148.00 ± 4.55 |
| STZ | 2.60 ± 0.25 b | 12.80 ± 0.66 b | 110.00 ± 4.09 b |
| STZ + Insulin | 5.00 ± 0.32 c | 6.80 ± 0.86 c | 142.20 ± 6.03 c |
| STZ + Aloe vera | 5.00 ± 0.45 c | 5.20 ± 0.58 c | 145.20 ± 5.32 c |
a Values expressed as mean ± SEM of five animals per group.
b P < 0.001 vs. the control group.
c P < 0.001 vs. the STZ group. One-way ANOVA; Tukey’s post-hoc test.
The thickness of molecular, purkinje, granular, and white matter layers at the apex of lobules (P < 0.001, P < 0.001, P < 0.001, and P < 0.01, respectively) and depth (P < 0.01, P < 0.001, P < 0.05, and P < 0.05, respectively) of cerebellum sulcus in the STZ group had significant reductions compared to the control group. Treatment with A. vera or insulin markedly improved the molecular, purkinje, granular, and white matter thickness at the apex of lobules (P < 0.05, P < 0.01, P < 0.001, and P > 0.05 for A. vera; P < 0.05, P < 0.05, P < 0.001, and P < 0.05 for insulin) and depth (P < 0.05, P > 0.05, P < 0.05, and P < 0.05 for A. vera and insulin) of cerebellum sulcus in the diabetic group (Table 2).
| Layers | Groups | ||||
|---|---|---|---|---|---|
| Control | Aloe vera | STZ | STZ + Insulin | STZ + Aloe vera | |
| Apex of cerebellum lobule | |||||
| Molecular layer | 247.56 ± 6.7 | 248.46 ± 5.7 | 198.86 ± 8.7 b | 230.73 ± 7.4 c | 229.9 ± 6.4 c |
| Purkinje cell layer | 25.15 ± 2 | 26.16 ± 1.5 | 19.36 ± 0.5 b | 22.72 ± 1.2 c | 23.87 ± 0.8 d |
| Granule cell layer | 195.75 ± 7.8 | 196.4 ± 9.8 | 145.2 ± 11.7 b | 190.21 ± 3.5 f | 184.15 ± 5.0 f |
| White matter layer | 59.25 ± 4.3 | 61.4 ± 3.8 | 49.65 ± 3.9 e | 56.12 ± 4.5 c | 54.25 ± 5.0 |
| Depth of sulcus | |||||
| Molecular layer | 332.56 ± 8.6 | 338.92 ± 7.2 | 298.86 ± 8.4 e | 322.78 ± 4.6 c | 320.75 ± 7.5 c |
| Purkinje cell layer | 31.56 ± 0.5 | 33.6 ± 0.2 | 24.6 ± 0.6 b | 29.77 ± 0.6 | 29.43 ± 0.5 |
| Granule cell layer | 205.20 ± 14.5 | 204.21 ± 15.6 | 181.42 ± 9.5 g | 196.48 ± 6.9 c | 195.54 ± 5.2 c |
| White matter layer | 98.20 ± 10.5 | 96.17 ± 9.6 | 75.64 ± 7.4 g | 92.48 ± 7.9 c | 91.45 ± 3.6 c |
a Values expressed as mean ± SEM of five animals per group.
b P < 0.001 vs. the control group.
c P < 0.05 vs. the STZ group. One-way ANOVA; Tukey’s post-hoc test.
d P < 0.01 vs. the STZ group. One-way ANOVA; Tukey’s post-hoc test.
f P < 0.001 vs. the STZ group. One-way ANOVA; Tukey’s post-hoc test.
e P < 0.01 vs. the control group.
g P < 0.05 vs. the control group.
5. Discussion
The results of this study showed that induction of diabetes caused hyperglycemia, decreased body weight, number of granular cells, and purkinje of the cerebellum after eight weeks; however, it increased the number of glial cells. Previous studies reported a wide range of central nervous system disorders in STZ-diabetic rats eight weeks after diabetes induction (9).
Treatment with A. vera gel not only improved hyperglycemia and decreased the weight of the diabetic animals, but also reversed these changes in the cerebellum cells at a level consistent with insulin therapy. Hyperglycemia and low blood glucose observed in diabetic animals is due to the effects of STZ on beta cells in the pancreas (20). Clinical and experimental studies have shown that A. vera can improve weight loss (21) and glucose (14, 22) in pre-diabetic and diabetic conditions. These effects may be due to the ability of A. vera to regulate expression of GLUT4, (23) protect the death or relative recovery of beta cells, (24) and regulate the secretion of insulin from remaining beta cells (25).
Bellamy reported that the induction of diabetes by STZ for eight weeks in rats caused cellular damage or cell loss in the cerebellum (13). Lechuga-Sancho et al. also showed that in diabetes, the activity of Caspase-3 cells continuously increases in all layers of the cerebellum, causing destruction, degeneration, and cellular changes in both glial (astrocyte, microglia) and purkinje cells, while the two above-mentioned cell groups form a common unit for the transmission of the synaptic information (26). During the diabetes period, the glucose consumption in the brain decreases, which causes the brain to become increasingly a vulnerable tissue (27). Previous studies have shown the destructive effects of diabetes and hyperglycemia on cell death by reducing IGF1 and P53 cell death transcription factors due to increased blood glucose levels (28).
Many clinical studies have also reported that type 1 diabetes can significantly reduce the density and volume of various brain regions (29, 30). Glial cells are always subject to any changes or disorders in the brain because in response to any changes in conditions such as diabetes, these cells alter their morphology and number to counteract these conditions (31). Astrocyte plays an important role in the transfer of glucose and its metabolites to neurons; therefore, changes in glucose levels after diabetes may be a possible cause of changes in the cerebellar astroglial population.
The activation and proliferation of microglia cells were reported in the cerebellum of diabetic animals in retinopathy, the acute myocardial infarction due to diabetes, and in the spinal cord of diabetic rats (32). In the present study, astrocytes and microglia in the diabetic rats showed changes in morphology and increased population in the cerebellum after eight weeks, while an increase in the population of glial cells after induction of diabetes by STZ was observed. In line with the findings of the present study, previous studies have shown that the first body immune cell in the brain, microglia, is readily activated to respond to any damage, dizziness, and metabolic disorder, and its number increases (33).
During immunodeficiency or cell death, microglia are activated to protect and repair the damaged tissue by removing the destruction caused by dead cells and facilitating the recovery process (34). Therefore, as shown in this study, the activation of post-diabetes activation of microglia in the cerebellum may be in response to cell death to provide potential damage control. On the other hand, studies have shown that microglial activation exacerbates degradation or possibly causes cell death through the production of various immune mediums (35).
Astrocyte also actively participates in a synaptic transmission associated with synaptic information processing modulation through astrocyte glutamate transporters that form triple synapses (36). As the primary cell of the cerebellar cell, purkinje cells play an important role in the coordination and learning of the motor system. Previous studies have reported that the inevitable loss of purkinje causes various motor disorders, such as autism, ataxia, and Huntington’s disease (37).
The mechanism underlying diabetes-associated structural disorders seems to be a multifactorial process. The evidence demonstrated that neural oxidative stress is of great importance in the pathophysiology of diabetes-associated structural disorders (21). It has been suggested that the neuroprotective effects of A. vera are partly mediated by preventing the decrease of endogenous antioxidants and suppression of lipid peroxidation markers (38). It has been confirmed that oxidative stress can lead to damage in the neural structural tissues (39). Recent evidence is indicative of the potential anti-oxidative properties of A. vera against diabetes-induced oxidative stress and oxidative mediators (40). Several reports have shown that the occurrence of oxidative stress in diabetic animals may induce structural disorders and neuronal cell death (41). It has been documented that treatment with A. vera can increase the levels of anti-oxidative defense in neural tissues by elevating superoxide dismutase, catalase, glutathione peroxidase, and reducing malondialdehyde (21). Furthermore, A. vera gel is known as a powerful antioxidant due to its components such as alkaloids, polysaccharides, , anthraquinones, coumarins, chromones, glycoproteins, flavonoids, vitamins (choline, acid folic, 1B,: 2B,: 6B,: 12B, C) and fat-soluble vitamins (E,: vitamin A precursor) (10). Hence, the amelioration of diabetes-associated structural disorders in cerebellum tissue may be partly due to the ability of A. vera to modulate the neural oxidative status; however, the mechanism of its action was not investigated in this study. Therefore, it is strongly recommended to include such measurements in future research works.
Previous reports have shown that A. vera reduces the expression of some cases such as kappa bacterial nuclear factor (NF-ҡB) and neuronal nitric oxide synthase (nNOS). Moreover, it alleviated inflammatory cell migration and edema hemorrhage, and neurons were partially protected from ischemic injury. Recently, a significant increase in NRF1 level was observed in the group treated with A. vera compared to the sciatic nerve ischemia/reperfusion group. According to the above-mentioned results, it was suggested that A. vera has effective neuroprotective characteristics against sciatic nerve ischemia/reperfusion injury via anti-inflammatory properties (11). It has been reported that A. vera protects the structure and function of mitochondria in PC12 cells (12). As inferred from the results of these studies, in the present study, the improvement of structural changes due to diabetes in the cerebellum by the A. vera gel might be related to its neuroprotective functions and anti-inflammatory activity.
In the current study, insulin also reduced blood glucose levels and improved histomorphometrical changes in the cerebellum of diabetic rats. Moreover, it was able to prevent the decline of purkinje cells and also prohibited the growth of microglia cells.
It has been suggested that insulin therapy has an antioxidant function in addition to controlling blood glucose (42). In this regard, it has been reported that insulin therapy in STZ-induced diabetic rats can reduce oxidative stress and also minimize cellular damage in the brain (12).
5.1. Conclusion
The findings of this study confirmed the improvement of the cerebellar tissue changes in diabetic rats following the use of A. vera gel at a level comparable to insulin. However, more investigations are required to determine the protective effects of A. vera gel against diabetes-induced cerebellum histomorphometrical changes.