1. Introduction
The abdominal cavity is a distensible compartment; however, it reaches a limit beyond which the pressure rises dramatically with the impairment of organ systems, a situation referred to as abdominal compartment syndrome (ACS) (1). Options for the closure of the abdomen under such conditions remain a debatable subject (2). Temporary closure techniques include simple packing, skin-only closure, Bogota bag, mesh coverage, Wittmann patch, and vacuum-assisted closure (VAC) (3). Each technique possesses its strengths and weaknesses; nevertheless, the Bogota bag is less expensive than most techniques, effective, and readily available (3).
In addition, patients presenting with ACS requiring surgical decompression have routinely been managed in intensive care unit (ICU) settings where the procedure might sometimes even be carried out (4). The ICU patients often have coexisting pathologies for which some form of organ support is required. However, routine intraabdominal pressure (IAP) measurements in ICU have been challenged (5), with an argument being made relatively for taking measures in those patients at high risk of intraabdominal hypertension (IAH). However, it can be presumed that in some healthy surgical patients with adequate physiological reserves, IAH or ACS encountered at laparotomy can be successfully managed with open abdomen (OA) techniques outside an ICU. This could have cost-benefit implications and improve overall quality care. Some surgeons distance themselves from decompressive laparotomy (DL) due to practical consequences, such as massive fluid loss from the OA, risk of sepsis, longer hospital stay, and fistula formation; nonetheless, DL has been proven to be a reliable approach to preserving organ function (6).
Not much room in the literature has been given to the non-ICU-based management of laparotomy-related ACS, managed primarily by simple OA techniques, serial clinical evaluations, and basic laboratory investigations. Herein, we present a case of the non-ICU-based staged operative management of a problematic anterior abdominal closure resulting from primary ACS in a previously healthy 23-year-old male during a 17-day hospital stay. This report has the potential to demystify the management of laparotomy-related ACS in low-resource settings.
2. Case Presentation
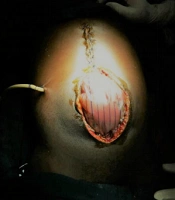
A healthy 23-year-old male was referred to an inner-city hospital with a 72-hour history of severe and worsening epigastric pain associated with a tensely distending abdomen. With a diagnosis of the acute surgical abdomen, a midline laparotomy was performed when he failed to improve on conservative treatment. The findings reported in the referral document included 3.0 L of ascitic fluid, tensely distended gall bladder, severe edema of the bowel, and retroperitoneum. The anterior abdominal wall could not be closed primarily on account of the significant edema of the retroperitoneum and bowel. The protruding bowel was subsequently covered with a large polypropylene mesh, and a Bogota bag was sutured to the fascia margin (Figure 1). The patient was subsequently referred to Komfo Anokye Teaching Hospital (KATH), Kumasi, Ghana, a tertiary center, for continued care in the ICU. He arrived with the vitals shown in Table 1.
| Variables | Values |
|---|---|
| Blood pressure (mmHg) | 130/90 |
| Pulse rate (beats/min) | 98 |
| SpO2 | 99% on intranasal oxygen at 5 L/min |
| Respiratory rate (cycles/min) | 27 |
| Glasgow Coma Scale score | 14/15 (M6E4V4) |
In KATH, the case was resuscitated and optimized over 2 days for a “second-look” surgery with the goal of thorough abdominal exploration and possibly further abdominal decompression and abdominal wall closure.
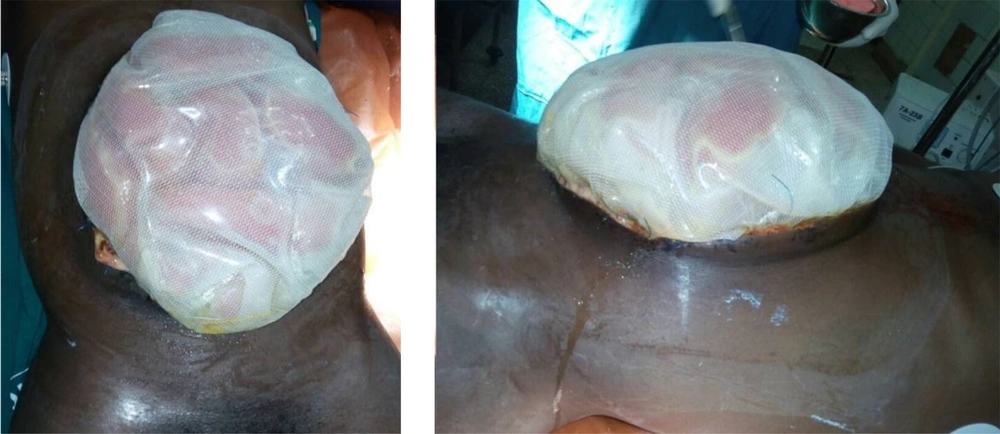
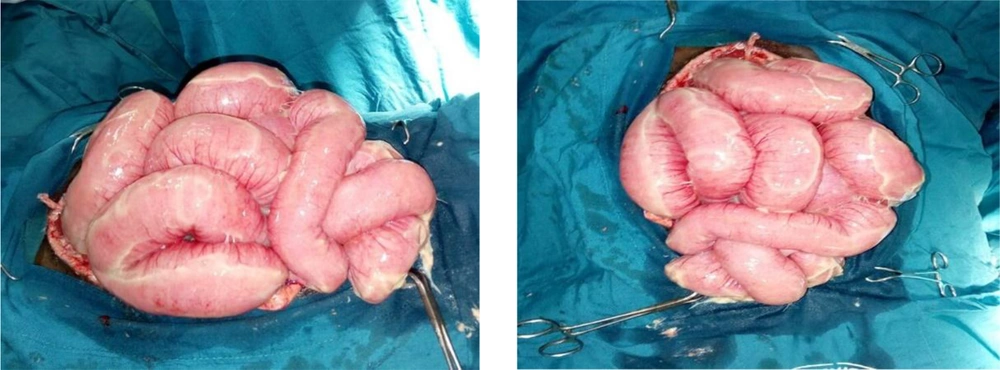
Intraoperatively, the gall bladder was observed to be autolyzed. The retroperitoneum and bowel remained significantly edematous (Figure 2). Decompressive enterotomy was conducted. The peritoneal cavity was generously lavaged, and a sub-hepatic drain was placed in the gall bladder bed. Since the fascia could not be primarily approximated because it was widely separated by the protruding viscera and had considerably retracted after the first surgery, a staged abdominal skin closure approach was decided on. The adjacent skin of proximal midline incision was closed craniocaudally using nylon-2 vertical mattress suturing to a safe lower limit which maintained a soft abdominal wall with normal vital signs and adequate urine output. For the distal aspect of the defect, a sterile drainage bag was improvised for a Bogota bag which was mounted and secured with non-absorbable sutures to the open fascia (Figure 3). One unit of whole blood was transfused intraoperatively. Sterile dressing was applied with circumferential abdominal wall loose bandaging.
The patient was returned to a limited-occupancy cubicle on the surgical ward, and postoperative management continued as follows: (1) regular clinical evaluation; (2) strict fluid input and output monitoring; (3) daily intravenous potassium maintenance administration; (4) adequate analgesia; (5) a full course of third-generation parenteral cephalosporin and metronidazole; (6) proton pump inhibitors.
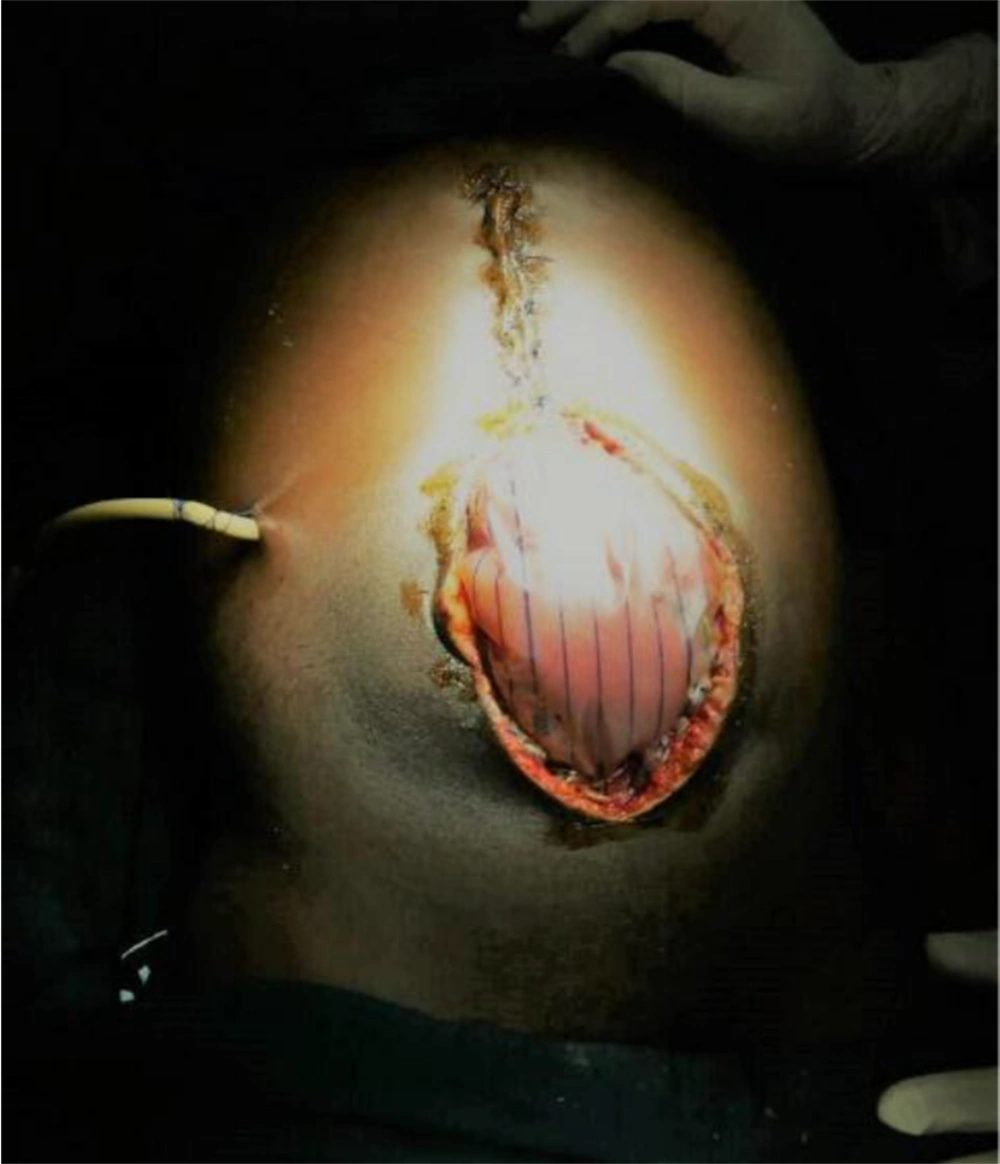
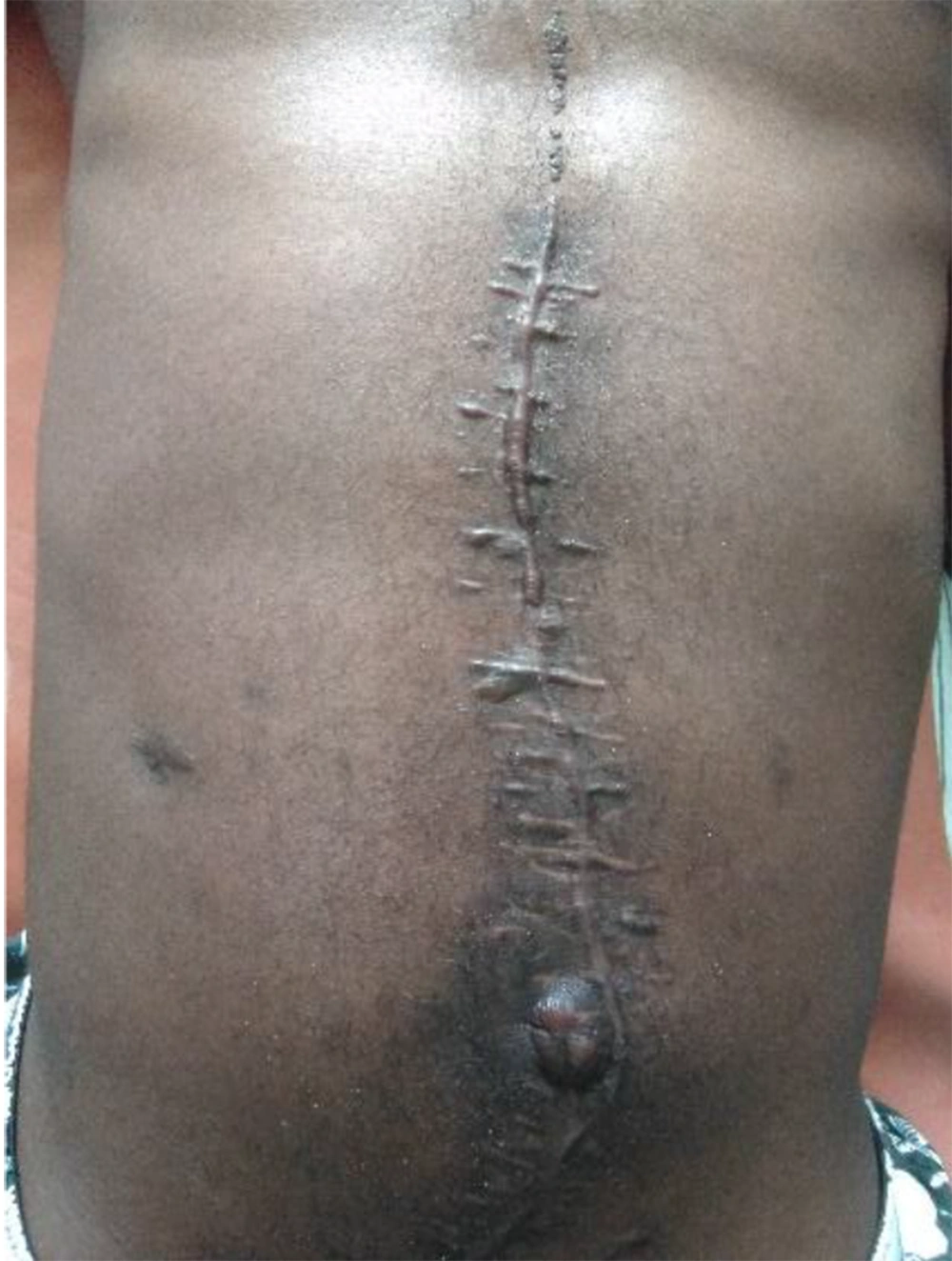
The abdominal drain recorded progressively smaller amounts of bloodstained bilious fluid. The abdominal wall gradually softened further, and full-length midline tension-free skin closure was possible on day 6 after the first stage of partial skin closure. Bowel sounds returned to normal within 24 hours, along with the passage of flatus, and the patient was allowed to start sips. Oral antibiotics (i.e., ciprofloxacin and clindamycin) were commenced on day 5 of the last surgery, and the patient continued to demonstrate good recovery. The abdominal drain was taken out on day 7 when output was less than 30 cc/24 hours. Afterward, his recovery proceeded unremarkably, and he was discharged on day 9 after the full-length closure. Follow-up continued at the outpatient clinic. All the sutures were removed on day 16 after satisfactory healing. As anticipated, he developed a large midline incisional hernia for which a polypropylene mesh repair was carried out 15 months later with good results (Figure 4).
3. Discussion
Beyond a full blood count, renal function, and blood cross-matching tests, not numerous other blood investigations are readily accessible in the typical Ghanaian primary health care setting, making it difficult to routinely employ sophisticated surgical prognostic scoring systems. Emergency laparotomies for intraabdominal sepsis or trauma are occasionally complicated by IAH or ACS requiring decompressive surgery as in the presented case. The consensus on abdominal wall closure techniques currently rests on the individualization of therapy based on sound surgical judgment (3, 4).
In the presented case, we described a scenario where two techniques were applied simultaneously at one stage of a multi-stage procedure. At the second laparotomy for the patient (and the first with the authors of this case in KATH), the skin was approximated proximally. When the abdomen started to become tense, the OA approach with a Bogota bag coverage was adopted for the distal midline wound (Figure 3). Because the drainage from the distal OA significantly reduced over 48 hours following the Bogota bag construct, along with improvement in the patient’s general condition, there was no indication for the bedside replacement of the bag before the subsequent surgery. It is reasonable to suggest that in combining closure techniques, a distally situated OA construct is preferred over a proximal one because gravity would further enhance drainage in the former. Additionally, an enterocutaneous fistula (ECF) developing as a complication of this procedure in the covered distal bowel generally fares better than an ECF that complicates a proximal bowel segment (7) in an OA procedure. Although the Bogota bag neither prevents fascia retraction nor drains abdominal exudates as effectively as VAC (3), it remains one of the least expensive methods currently available. Moreover, the Bogota bag could be immensely beneficial for rural and suburban surgical practice when well appropriated. In the absence of the sterilized drainage bag used in our case, other materials reported to be used as improvisation include pre-sterilized 1 - 3 L soft infusion bags (3).
Full-length midline skin-only closure employed at a single stage in the operative decompression of ACS has been associated with recurrent ACS in 13 - 36% of cases (8) and would have been hazardous to employ in this patient who was beginning to show signs of a tensed abdominal wall midway through the skin closure. In the absence of a dedicated ICU, the management of surgical decompression for ACS on the regular ward in such a patient by alternative methods, such as simple packing of the OA, daily dressing change, and bedside abdominal lavage, despite having demonstrated good morbidity and mortality profiles (9, 10), might not be a safe option due to the considerable risk of contamination and sepsis from environmental microbes.
Clinical examination has not proven very reliable in the detection of IAH and ACS (11, 12); however, because routine measurement of IAPs and transducers to carry out this is practically nonexistent in most West-African hospitals, it makes the judicious use of clinical acumen very crucial in these settings. In this report, we draw attention to the possibility of managing a selected case of a challenging abdominal wall closure using available materials to avert ACS. This is the result of meticulous and serial clinical evaluation. An admission Quick Sequential Organ Failure Assessment score of 2 (Table 1) in this patient was aptly an indication for increasing the frequency of monitoring all the same (13).
Serial monitoring aids the early detection of clinically unstable patients or patients likely to further deteriorate and might need to be transferred for ICU care elsewhere. Again, depending on patient factors, the nature and severity of the pathology, complications, and comorbidity profiles, the anterior abdominal wall reconstruction outcome could be quite satisfactory (Figure 4) (14). What remains for further research is the development of a set of reliable clinical parameters or criteria to select suitable candidates for this type of approach to laparotomy-related ACS management. This select population of patients would likely include healthy young individuals without significant comorbidities presenting with acute abdominal emergencies that require laparotomy.
Additionally, in the process of undertaking this OA technique to avert post-laparotomy ACS, as described in the reported case, devising a pragmatic set of clinical monitoring parameters would allow for the identification of patients for whom the treatment course could safely proceed outside the ICU while advancing a cost-effective allocation of limited resources without compromising quality patient care. A helpful starting point includes the close observation of the changes in clinical signs associated with ACS, such as abdominal distension, refractory hypoxaemia, refractory oliguria, and those signs resulting from a raised intracranial pressure (1). The aforementioned items should be carried out together with the appropriate management of whatever is the primary underlying pathology. For example, underlying acute pancreatitis treatment should include ongoing intravenous fluids, analgesia, nutritional support, and prophylactic antibiotics if indicated (15).
3.1. Conclusions
Herein, we described the staged abdominal wall closure in a 23-year-old male managed by a staged OA technique to avert ACS resulting from massive retroperitoneal and bowel edema. The highlight of this report is the successful 17-day in-hospital management of the patient in a limited-occupancy surgical ward without the need for an ICU but relatively employing serial clinical evaluation and regular monitoring, which formed the bedrock of his care. This level of care can adequately be offered in most Ghanaian district and regional hospitals for selected patients. Again, under the appropriate conditions, as in this patient, skin-only closure and Bogota bag coverage can be used concurrently at the same stage of the temporary closure. The Bogota bag might not need frequent bedside replacement.