1. Background
The Urinary tract is the path that carries urine out of the body for eliminating waste & extra water [1]. That these infectious involving the bladder, the kidneys, the ureters. When the lower urinary tract infection affects simple cystitis is called (infection in the bladder) and when it effects on pyelonephritis are (infection in the kidney) [2]. Urinary tract infections are caused by microbes such as E. coli [3]. The world health organization reported an outbreak of excessive urinary tract infection that caused by E. coli as this is resistant to a high range of antibiotics [4]. The Infection in the kidneys is a kind of UTI that often created by Escherichia coli [5]. Urinary tract infection when bacteria enter the urinary tract that can be distributed. Since the urethra in women is shorter than the men, bacteria can more easily into the urinary tract and cause symptoms. However, sometimes other bacteria are responsible. In more studies Uropathogenic Escherichia coli (UPEC), caused Urinary tract infection (UTI) especially in women, the Recurrences are common [6, 7]. In recent years Urinary tract infections are developing & are the second most common infection in the world [8]. Men are less than women involved to urinary tract infections, because; men have bigger urethra in their body [9]. UTI is a disease which, created in the woman or man and can transmitted it together (Venereal disease) [10]. Women in usual condition involved to UTI more than pregnant women because the anatomical revises happened in kidney of pregnant women [11, 12]. Urinary tract infection (UTI), caused by E. coli bacteria, in 85% of the women and children who were urinary tract infection leading to kidney failure seen death [13]. In recent years, resistance to antibiotics is a great public health problems posed in the world, Resistance to antibiotics as a threat to human health, which is on the rise. Multi drug-resistant E. coli infections increased annual and it is a serious problem [14, 15].
Nanoparticles are attributed to a group of atoms with the size of 1 - 100 nanometers the basic advantage of NPs is that they are easy to synthesize by different of bacteria [16]. Their surface to improve their application of NPs [17]. In order to consider the size and shape of NPs, decreasing factors, etc., have been used in the preparation of NPs [18]. The most of important dramatical feature of Zno nanoparticles is their small size and large surface area of the material that cause to increase the chemical and biological properties and the antimicrobial activities [19]. Zno nanoparticles have catalytic, magnetic, optical and biological (antimicrobial) properties Among nanoparticles, ZnO nanoparticles attract the researchers’s attention for having specific chemo-physical properties [20]. These nanoparticles are able to bind to membrane of bacteria cell and decrease the integrity of the bacterial membrane [21].
2. Objectives
In this research, we tried to use an appropriate technique for find out the UTI infection in E. coli and applying the nano-biologically methods such az ZnO nanoparticles. E. coli bacteria is a gram negative bacteria that we considered the effect of ZnO nanoparticles against it and also, in this research we compare the antibacterial effect of ZnO nanoparticles with other researches, with testing these Nps on E. coli bacteria that caused Urinary tract infection.
3. Methods
3.1. Patients and Determinants
In this experimental study During 8 months in 2014, at first were used urine Samples from 257 women with UTI that infected by E. coli, then we used from antibiotic disks for identification the MDR E. coli such as: cefazolin, Tobramicin, Cefotaxime, Co-Amoxiclav, Amoxicillin, Cotrimoxazole, Cefexime, Cloxacillin, Cephalexin, Ciprofloxacin, Ampicillin. 91 samples were isolated from 257 women urine samples that, there were multi drug resistance E. coli. This experimental study has been done in Isfahan city, Iran totally with 91 numbers of women suffering from Urinary tract infection in groups of different ages from laboratory of alzahra hospital. In alzahra hospital laboratory for identification the E. coli utilized from biochemistry medium specially such as Urea, MR-VP, SIM, Citrate, TSI & Catalase testing. The Urine samples were collected applying Midstream clean catch method and cultured in blood agar and EMB medium using standard loop then incubated for 24 hours at 37°C. Also, this research utilizes standard E. coli PTCC 1399 provided in health education and research center in Isfahan as the control [22].
3.2. Preparation of E. coli Cultures and Evaluation of Antibacterial Activities
The select cultures for E. coli were Luria Bertani (LB) broth and nutrient agar, it’s finally light absorption in the wave length to 0.08 - 0.1 requited. In this case, the cell suspensions used for antibacterial activity contained 1.5 × 108 CFU/mL, which is a bacteria cell concentration, is standard antimicrobial effects.
3.3. Properties of ZnO Nanoparticles
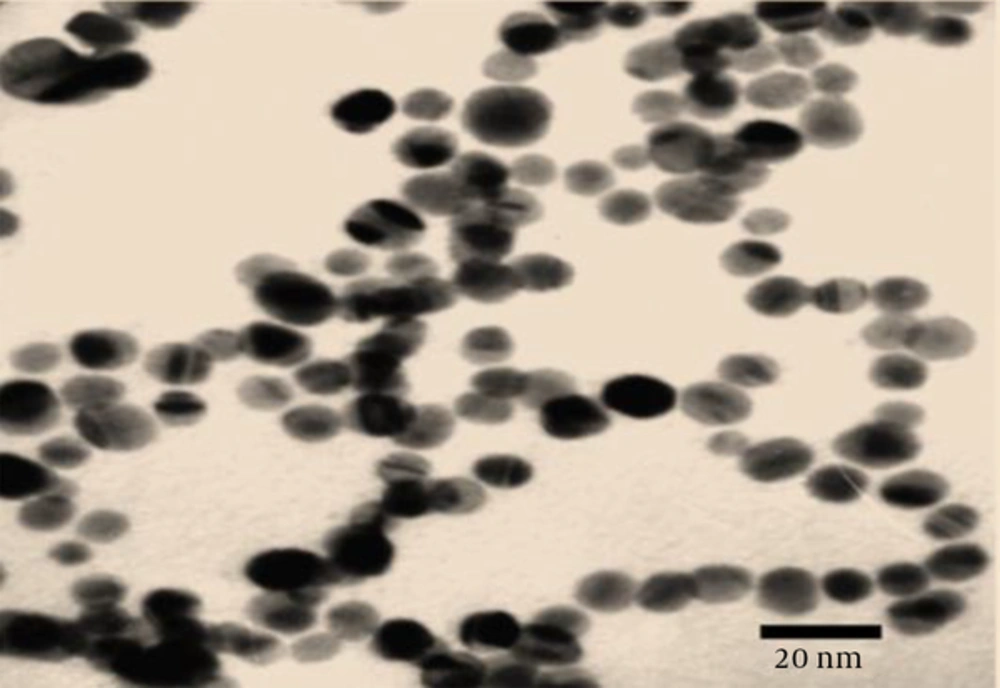
200 mL of colloidal ZnO nanoparticles in colloidal solution at 20 nm and a concentration of 100 ppm to a spherical shape , 200 mL of ZnO nanoparticles bought from neutrino company in Tehran, Iran and dilutions in different concentrations was prepared such as: (10, 20, 40) ppm .
3.4. Diffusion Method
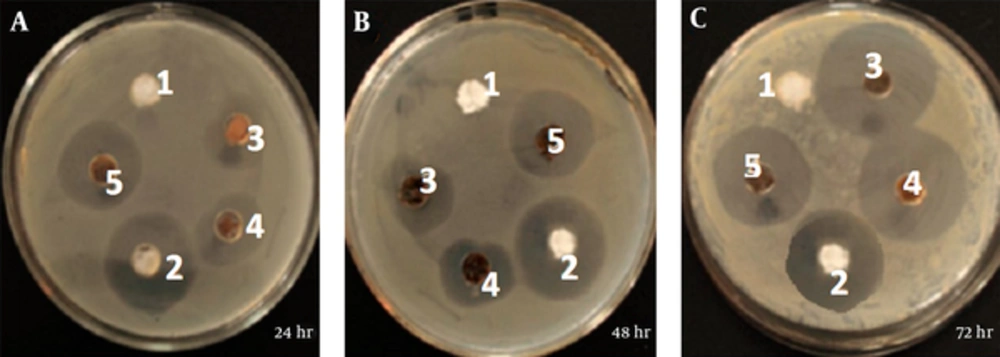
The 91 isolated of E. coli (the urine samples) were prepared, with sterile cottony swab on the surface of the Luria Bertani (LB) and nutrient agar medium was growing steadily. 100 microliters of different concentrations of ZnO nanoparticles produced separately added to each well. The 100 mL concentrations of 10, 20 and 40 ppm prepared from ZnO nanoparticles were added to each well. Sterile water as negative control and the Gentamicin disk 25 mg (Himedia India Company) was used as positive control. The inhibition zone diameter was measured in millimeters every days. Also in the Muller Hinton agar was steadily growing. The end of the Pasteur pipette, pit to a diameter of 6mm was created in vitro. The experiment was repeated three times for each sampleand inhibition zone diameter in milimeter after 72 hours at 37°C was measured [23].
3.5. Microdilution Method
MIC material used was determined by microdilution method. In these methods, under aseptic conditions, For Nano ZnO particles: 100 mL of dextrose broth sabouraud environment sterile microplate was added to all wells. Then were added 100 mL of dilution and 100 ppm of ZnO nanoparticle then were added dilution made with ZnO nanoparticles 10, 20 and 40 ppm. We have positive and negative control. In the end, all the wells 10 mL of fungal suspension of each isolate were added. Microplates are incubated for 24 hours at 37°C. After incubation, the wells were checked for turbidity. Staining that growth was not in it was considered as the MIC. The wells that no growth of bacteria observed, obtain the MBC amount [24]. Finally, The data were analyzed by spss 16 version software and evaluated mean and SD amount and differences plotted charts and graphs with Excel 2013 software, P value of < 0.001 was considered statistically significant (Figure 1).
4. Results
During 8 months, 257 samples were examined, that 91 samples were reported positive. The basic antibiotics resistant to Escherichia coli were 16/91 percent to cefazolin, 40/80 percent to cefexime and 80/28 percent to cefotaxime Table 1. The results showed that ZnO nanoparticles have a good antibacterial effects against Urinary tract infection and can be used to treat infections of E. coli, it is recommended that further research considered the effects of different infections of Urinary tract infection in In vitro condition. Diffusion method results from the impact of the ZnO nanoparticles on samples of E. coli. Figure 2 shows the diameter of the inhibition of E. coli isolates the impact of the ZnO nanoparticles in different concentrations and incubated after 72 hours at 37°C. The diameter of the growth of ZnO nanoparticles 20 nm on isolated, zero to 12 mm. Most average inhibition zone diameter ZnO nanoparticles at concentrations (10, 20, 40) ppm, respectively, 4, 7 and 12 mm (Table 2).
| Semi-Sensitive | Resistance | Sensitive | Antibiotics |
|---|---|---|---|
| 2 | 16/91 | 84/6 | Cefazolin (E) |
| 35/20 | 94/52 | 71/26 | Tobramicin (TOB) |
| 6 | 63/62 | 37/31 | Co-amoxiclav (AMC) |
| 5 | 28/80 | 72/14 | Cefotaxime (CTX) |
| - | 47/76 | 53/23 | Amoxicillin (AMX) |
| 2 | 71/72 | 29/25 | Cotrimoxazole (SXT) |
| - | 40/80 | 60/19 | Cefexime (CFM) |
| 33/3 | 67/66 | 30 | Cloxacillin (CX) |
| 22/5 | 70 | 78/24 | Cephalexin (CN) |
| 02/11 | 98/50 | 38 | Ciprofloxacin (CP) |
| 49/8 | 51/71 | 20 | Ampicillin (AM) |
| Zno Nanoparticles | Mean ± SD |
|---|---|
| 10 | 4.65 ± 1.87 |
| 20 | 7.24 ± 3.15 |
| 40 | 12.31 ± 6.84 |
4.1. The Toxicity Mechanisms of Zno Nanoparticles Against Escherichia coli
In our research, the results published in the well showed that the diameter of bacterial growth inhibition increased with increasing concentrations of ZnO nanoparticles, The ZnO nanoparticles (20 nm) dilutions against MDR E. coli were examined by well agar diffusion in Luria Bertani broth and then were measured MIC and MBC. The results showed that ZnO nanoparticles have a bacteriostatic effect and can damage the cell membrane and eleminated the bacterial cell. The mechanism of ZnO nanoparticles depend on surface area of material (ZnO), type of bacteria for instance (the fast are more sensitivity than slow growing bacteria to nanoparticles growing bacteria), physiochemical properties of ZnO nanoparticles (P < 0.001).
4.2. MBC and MIC Results
The MBC is the lowest concentration that to kill the most of the bacteria. The MIC is the concentration that observed the turbidity of bacteria [25]. MBC and MIC Results showed by microdilution method on isolated from patients with urinary tract infection. The ZnO nanoparticle samples were evaluated according to this method. The (MIC) and (MBC) of ZnO_NPs were respectively 15.73 × 9.13 and 34.61 × 26.58 (Table 3 and 4).
| Samples | MIC, ppm | MBC, ppm |
|---|---|---|
| Escherichia coli | 15.73 × 9.13 | 34.61 × 26.58 |
| Escherichia coli (Standard Sample) PTCC 1399 | 8.67 × 5.17 | 10.41 × 12.49 |
| Nanoparticle | Size (Average) | Microorganisms | MIC | Proposed Mechanism | Reference |
|---|---|---|---|---|---|
| ZnO | 13 nanometer | Staphylococcus aureus | decreased 95% in 80 μg/mL | Reactive Oxygen Species (ROS) inhibition | Reddy et al. [26] |
| ZnO | 60 nanometer | S. aureus | decreased 50% in 400 μg/mL | Reactive Oxygen Species inhibition | Jones et al. [27] |
| ZnO | 40 nanometer | S. aureus, Escherichia coli | Both species reduced 99% at 400 μg/mL | disrup the integrity of cell Membrane | Nair et al. [28] |
| ZnO | 12 nanometer | E. coli | decreased 90% in 400 μg/mL | damage the Membrane | Padmavathy and Vijayaraghavan [29] |
| ZnO ions | N/A | Pseudomonas aeruginosa, S. aureus, Candida albicans | decreased 100% in 1917, 9, and 39 μg/mL, respectively | Reactive Oxygen Species inhibition | McCarthy et al. [21] |
a**In Table 4 reported the antibacterial properties of ZnO nanoparticles against variety microorganisms and fungus.
5. Discussion
According the result of this study, Using ZnO nanoparticles with 20 nanometer diameters have a debateable antibacterial effect on 91 samples of Escherichia coli isolated from Urinary tract infection and its function has been proved. the current study halting effect of ZnO nanoparticles on microorganisms experimented in different densities was observed. The mean diameter of inhibition at concentrations of 40 ppm have a high inhibition zone. Based on the attained results anti-bacterial activity of ZnO nanoparticles was depended on concentration. The growth reduction of S. aureus and E. coli were revealed to ZnO nanoparticles of lowing size. However, higher concentration was need to decrease viability of different bacteria at 24 hours in size of 40 nm of ZnO nanoparticles [21, 28, 29]. In the event that, In our research we used ZnO nanoparticles in size 20 nm and in 40 ppm concentration after 72 hours, have a reduce viability for E. coli .Soltani and et al. studied that the treatment process by ZnO nanoparticles can reduce the length of treatment course and implications caused by medication in which these results is the same of our findings in my research the size of ZnO nanoparticles was 20 nm. During the present study, different concentrations of ZnO nanoparticles were tested to dicover the best concentration that can have the most effective antibacterial property against the E. coli. This data have consistant with our studies, dealing with the antibacterial effects of Nano-materials. Zhang et al. studied the antibacterial properties of ZnO nanoparticles against gram positive bacteria with nano particles & with out using from antibiotics against this bacteria. The authors gain acceptable results with effective of ZnO_NPs [25]. The effect of nanoparticles was experimented on two bacteria species and then was tested in cell membranes of bacteria. An increased antimicrobial activity of ZnO_NPs on E. coli bacteria was studied at the 18h, the diameter was increased from 2 μm to 45 nm to 12 nm, and the higher antibacterially effect of these NPs was attributed to smaller size of nanoparticles [12, 24]. In our research, the results published in the well showed that the diameter of bacterial growth inhibition increased with increasing concentrations of ZnO nanoparticles that have Consistent with our research. Liu et al. reported, Antibacterial effects of ZnO_NPs against E. coli O157:H7 showed that these NPs can attach to cell membrane by electrostatic reaction and disruption the integrity of cell membrane then kill the E. coli O157:H7 cells [30]. This study have illustrated the antibacterial feature of ZnO nanoparticles .It is clear that these nanoparticles have high prevent effect on life of different bacteria species even Listeria monocytogenes. It is observed that zinc oxidenano ZnO NPs have penetrated the membrane of bacteria and have caused damage by interacting with phosphorus and sulfur compounds such as DNA. Nanoparticles have a relatively large surface area and have more contact with bacteria [31]. Wahab et al. studied, the spherical shapes of ZnO-NPs exhibit the vast antimicrobial effects against pathogens bacteria, this structure of spherical nanoparticles in various studies which the antibacterial property of ZnO-NPs confirmed, similar to our researches. The acceptable features of ZnO-NPs against (E. coli, S. aureus, P. aeruginosa & B. subtilis) verified, even for treat the cells that cancered [32]. Amna Sirelkhatim et al. studied that when (ZnO-NPs) have small size and the large surface that caused to much movement in human body and promote the chemical, biological, medical and catalytic activities [33]. The antibacterial effects of ZnO nanoparticles (5 and 100 nm in size) at concentrations of 12.5, 25.0, 50.0, and 100.0 mg/mL were determined using the well diffusion method in vitro. Maximum inhibitory concentration of ZnO nanoparticles was determined as 25 mg/mL. A significant relationship was found between antibacterial activity and ZnO nanoparticles concentration. It may be claimed that treatment with polypropylene filters coated with ZnO nanoparticles (5 nm) is an effective process for controlling bacterial growth and eliminating E. coli [34].