1. Background
The removal of bacteria from water is an extremely important process for drinking and sanitation systems especially against concerns on growing outbreaks of water borne diseases [1]. In the United States, only between 2003 and 2005 there were four reported waterborne disease outbreaks attributed to pathogens in drinking water affecting 282 people [2]. Conventional methods for disinfection of water are dependent on chemical agents, that are ineffective against cyst-forming protozoa such as Giardiaand Cryptosporidium and also these methods often produce harmful by-products.
Nanotechnology is considered as a new generation of technology that can have a great impact on economies through new consumer products, manufacturing methods and materials usage [3]. This technology can lead to cost effective and high performance water treatment systems [4]. By the use of nanotechnology, implementation of oligodynamic nanoparticles for water disinfection is being explored. Oligodynamic nanoparticles based disinfection includes the use of metals such as silver, gold, zinc, tin and copper due to their antimicrobial properties. Besides their oligodynamic nature, they also possess catalytic properties [5].
In 1985, Matsunaga et al. [6] reported the antibacterial properties of TiO2 particles for the first time, which attributed to the high redox potential of the surface species, affording non-selective oxidation of bacteria. Hashimoto et al. [7] have reviewed the antibacterial effects and detoxifying actions of TiO2 photocatalyst on ceramic tiles. Since then, many photocatalytic inactivation studies have been conducted on a wide spectrum of organisms including cancer cells, phages, viruses, bacteria, fungi, algae and protozoa. Large band gap semiconductors, such as TiO2, SnO2, SiO2 and ZnO are suitable photocatalytic materials [8-10]. Among these Tin oxide (SnO2) is an important n-type metallic oxide semiconductor with wide band gap (3.6 eV). Because of its unique electronic, optical, electrochemical and catalytic properties, SnO2 were extensively used in solar cells, transparent conducting electrodes, solid-state sensors, rechargeable Li batteries and optical electronic devises [11, 12]. The conductivity and optical properties of SnO2 are largely dependent on the particle size and shape of the nanocrystallites [13-15]. To obtain quantum size SnO2nanocrystallites, the sol-gel method [16] and hydrolysis of SnCl22H2O were carried out [17]. Recently, SnO2 Quantum dots were also fabricated using hydrazine hydrate as the mineralizer instead of NaOH by a hydrothermal route [18].
However,it is still a great challenge to fabricate the nanostructure SnO2 with controlled-size and tunable shapes by wet chemical methods [19].
2. Objectives
In this study, the solvothermal method was employed to synthesize SnO2 nanoparticles with spherical morphology in the absence of templates or structure-directing agents under mild conditions. The nanoparticles used to in the inactivation efficiencies for two microorganisms under UV irradiation and dark conditions.
3. Materials and Methods
3.1. SnO2 Powder Preparation
In this experimentalstudy, the solvothermal method was employed to synthesize SnO2 nanoparticles.
In a typical process, desired amount of SnCl45H2O and 1.2 g NaOH were mixed with 20 mL de-ionized water under magnetic stirring for 10 minutes. Twenty milliliter absolute ethanol was dropped slowly into the solution to make the white precipitation. The mixture was stirred for 24 hours and then the whole mixture was transferred into 50 mL Teflon-linked autoclave. After the reaction completed, the resulting solid product was filtered and washed several times by de-ionized water, absolute ethanol and finally dried at 120°C in air for 24 hours.
3.2. Antibacterial Performance
The antibacterial activity of the synthesized SnO2 nanoparticles was evaluated using bacterium as per colony count method. To examine the bacterial growth rate, cultures (bacterial strain) were grown in the nutrient broth medium (NB) supplemented with SnO2 nanoparticle colloidal suspension at concentration of 20 ppm. The SnO2 nanoparticles were added into 10 mL NB medium which was added with 200 μL bacteria at a concentration of 1.5 × 106 CFU (colony forming unit). The culture of nanoparticle free medium as blank was grown under the same reaction conditions and was used as a control. The samples and blank were illuminated with four 8 W lamps (Philips UV-A, k = 365 nm), at room temperature for 0.5, 1, 2, 3, 4 and 5 hours, respectively. The temperature of the culture medium was controlled using the water circulation through the reactor. During each time period, the sample containers were placed horizontally onto a shaker platform and agitated at a speed of 225 rpm. After catalyst centrifugation and separation, 100 μL of each bacteria suspension was dispersed on the Nutrient Agar medium. The number of colonies forming units (CFUs) were counted taking into account the dilution factor after 24 hours of incubation at 37°C. The respective data are the average of values obtained from triplicate runs. The catalysts under consideration have not shown a decrease in their photo induced micro biocide efficiency when the antimicrobial experiments were repeated three times using the same samples. No significant decrease in CFU in nanoparticle free medium observed after UV light exposure. This indicates that the photochemical inactivation was negligible under UV-A irradiation with low energy.
3.3. Sample Characterization
To determine the crystal phase composition of the prepared photocatalysts, X-ray diffraction (XRD) measurement was carried on a Bruker, D8 ADVANCE XRD diffraction spectrometer with a Cu K α line at 1.5406° A and a Ni filter for an angle range of 2θ = 20 - 70°. To determine the band gap energy of the photocatalysts, UV-vis spectroscopy measurement was carried out using a double-beam spectrophotometer Cary 500 scan. UV-vis spectrophotometer operated over the range of 190 - 800 nm at a resolution of 2.0 nm. Philips XL30 scanning electron microscope (SEM) measurements were also used to investigate the morphology of the samples with an accelerating voltage of 17 kV.
4. Results
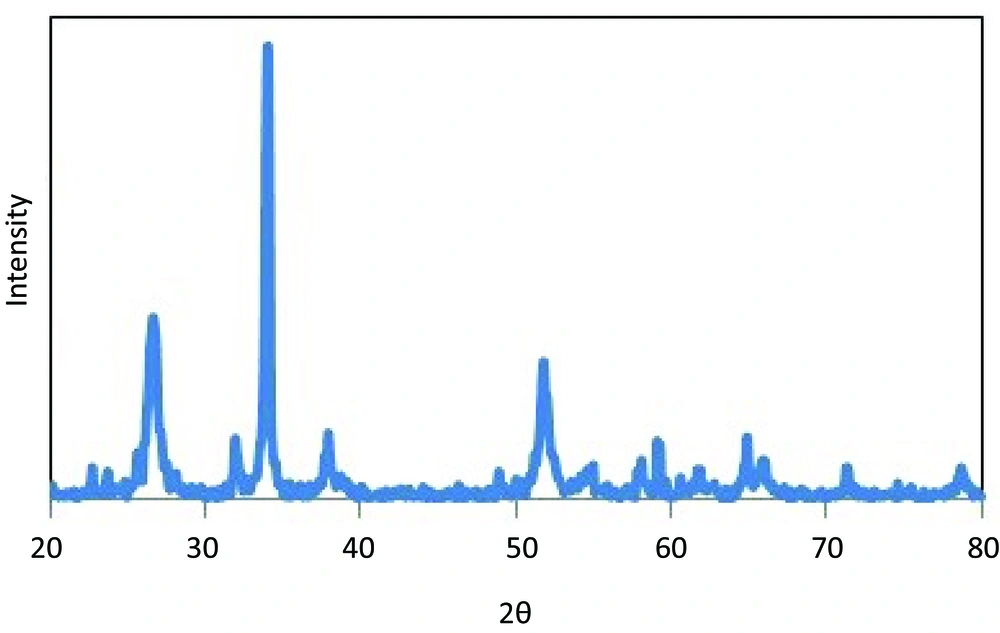
The XRD pattern of SnO2powder is shown in Figure 1. The patterns of SnO2 powder display the (1 1 0), (1 0 1) and (2 1 1) diffraction peaks of the tetragonal rutile structure for SnO2 crystalline phase (space group: P42/mnm, a = 0.4756 nm, c = 0.3175 nm) at 2θ about 26.5°, 34° and 52.1°, respectively. No remarkable shift and no characteristic peaks for impurity, such as SnO, ZnSnO3, and Zn2SnO4 in diffraction peak were detected. The surface energies of SnO2follow the trend (110) < (100) < (101) < (001), indicate the c-axis to be the preferential growth direction [20].
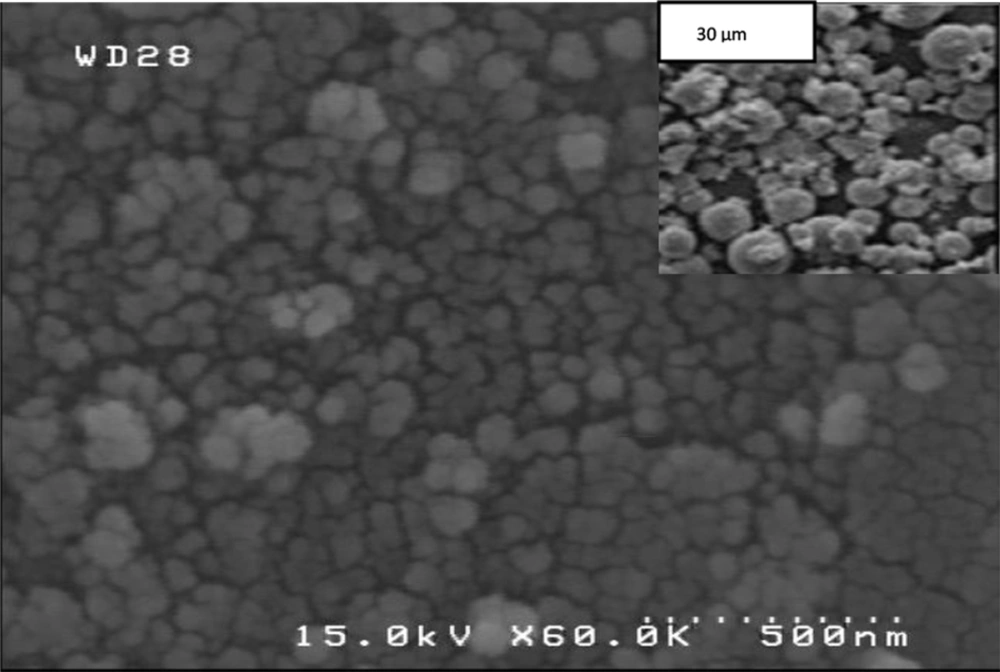
By conducting experiments in solutions, the morphology is therefore dictated by the crystal symmetry as well as by the surface energy in the aqueous environment and thus the most stable crystal habit is directly generated without template or surfactant. Further structural characterization of the SnO2 powders was performed by SEM. It was found that the hydrothermal treatment led to synthesized spherical nanoparticles with 66 nm diameters (Figure 2). The morphology of the final products may be explained by both crystal growth and nucleation theory in which the synthesis is separated into two steps: nucleation stage and crystal growth. In the nucleation stage, Sn(OH)62-nucleus are created which decompose to SnO2.At lower hydrothermal temperature instead of crystal growth stage, the greater the number of nuclei was created in the solution where nuclei attracted species and other nuclei around them to form spherical shapes.
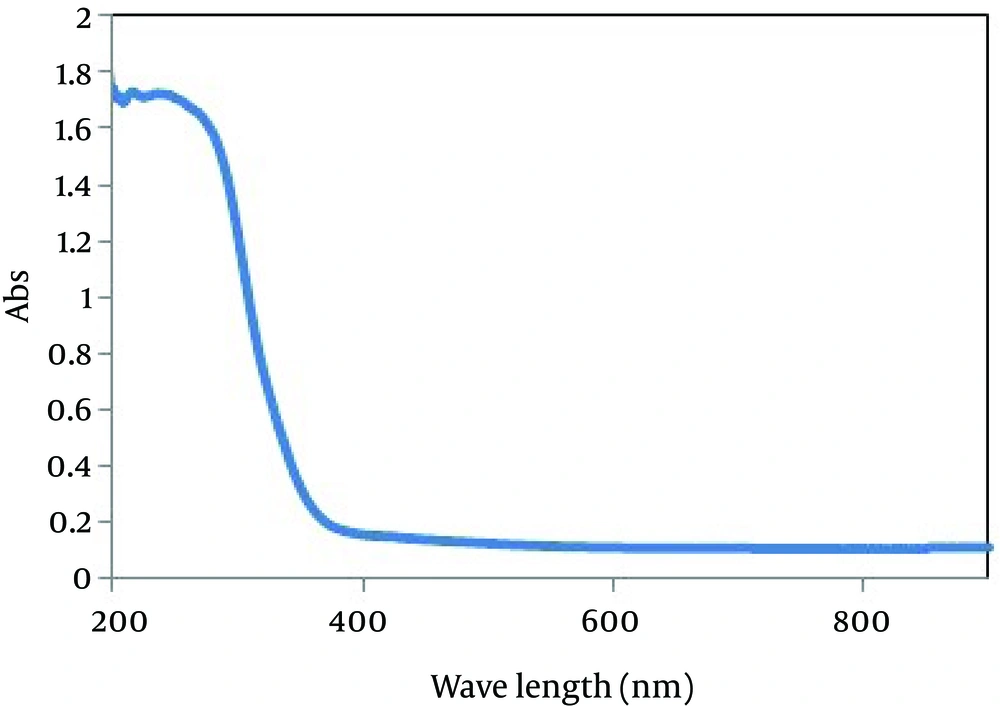
UV/vis spectroscopy was used to characterize the optical adsorptions of SnO2 sample as shown in Figure 3. The fundamental absorption edge of the sample corresponds to electron transitions from valence band to conduction band and this edge can be used to calculate the optical band gap of the sample. The optical transition of SnO2 crystals is known to be a direct type [21]. In this case, the absorption coefficient is expressed [22]. The band gap energies (Eg) were calculated on the basis of the corresponding absorption edges and by extrapolating the horizontal and sharply rising portions of the curve and defining the edge as the energy of the intersection (not shown here).
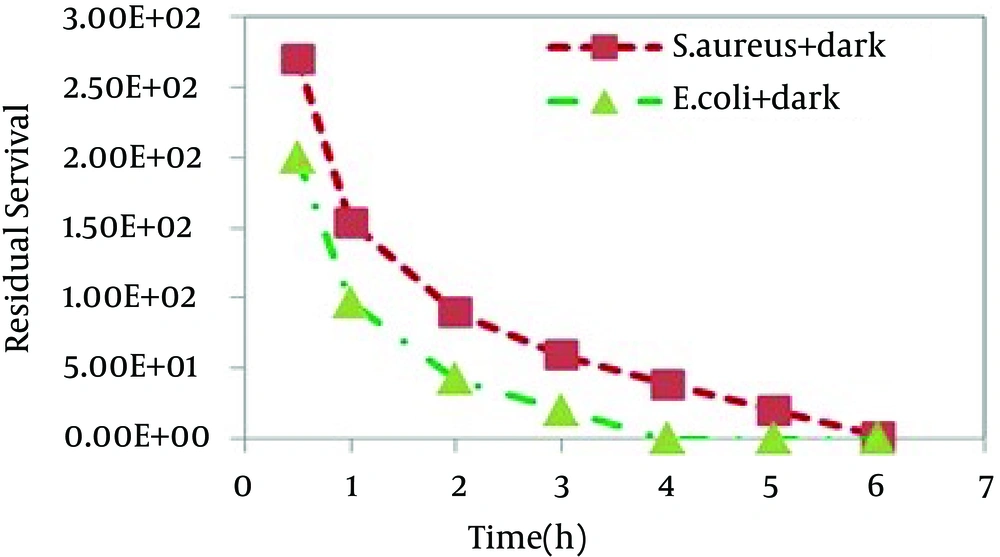
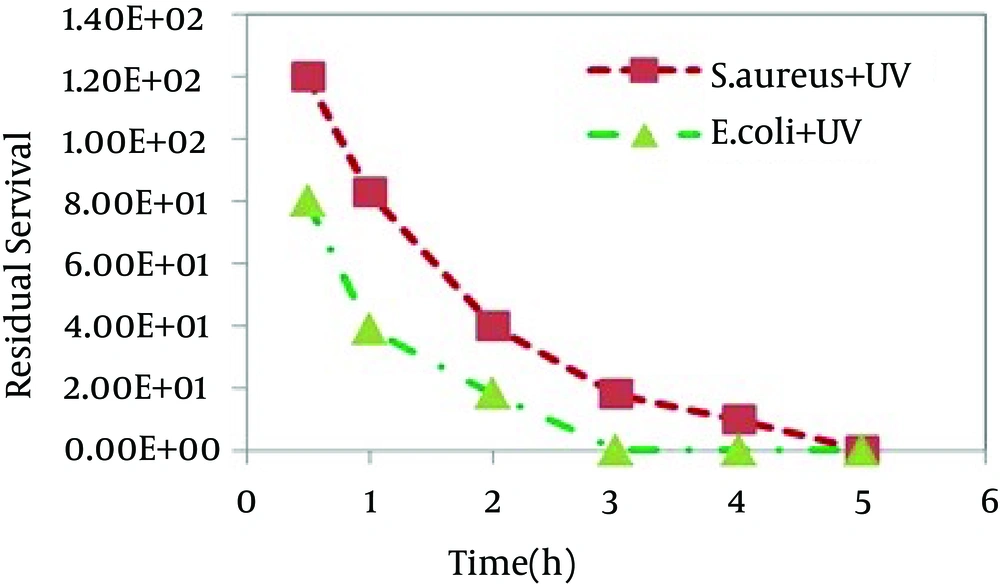
E. coliand S. aureuscells were exposed to different samples for up to 5 hours to assess any bactericidal effect of the catalyst under UV light and dark conditions. Figures 4 and 5 show the relative number of bacteria survived after the antibacterial experiments.
5. Discussion
In this study it was found that SnO2 nanoparticles had a good antibacterial activity against Gram-positive and Gram-negative bacteria. The antibacterial effect of the synthesized photocatalyst towards E. coli and S.aureus was investigated by initially performing controlled experiments. Two individual runs were performed as control experiment. Blank control experiments (bacterial solutions without any catalyst) under dark and UV light irradiation were performed and no significant decrease in CFU of nanoparticle free medium were observed. In other controlled experiments, bacterial cultures were incubated with nanoparticles in dark. Similar experiments as mentioned above were performed by incubating bacterial cultures with nanoparticles under UV illumination. The larger band gap of SnO2 sample enhances its UV-light absorption ability resulting in the formation of highly reactive oxygen species (ROS) which are responsible for bacterial cell damage. Therefore, UV illumination improved SnO2 sample activity compared to dark condition. It is worthy to note that in the absence of UV light, all samples showed bactericidal activity. In contrast, nano-TiO2, a common kind of bactericidal material, is photoactive and requires light irradiation in order to exhibit effective bactericidal activity.
The nanoparticle morphology, surface area, surface charge, its material solubility and degree of aggregation in a particular system being studied will strongly influence the availability of nanoparticles for uptake into cells and resulting toxicity in aquatic systems [23, 24]. The following factors may affect the antibacterial activity of SnO2 powders: 1- Highly reactive oxygen species (ROS) generated including hydroxyl groups (OH), superoxide anions (O2-) by single-electron reduction which does not require UV irradiation [25, 26]. However, photo catalytically induced oxygen bacterial cells. 2 -pH: in our work, pH of the powder samples dispersed in physiological saline was 7.5, irrespective of the sample. 3- Membrane dysfunction [27]. 4- Nanoparticle internalization [28]. Species improve the bactericide effect of UV-illuminated samples. It has been generally accepted that likely mechanism of antibacterial photoactivity of metal oxides is due to interaction with highly reactive oxygen species (ROS) which would result in oxidative damage of the cell membrane or inside the cells [29, 30]. These ROS can include hydroxyl groups, superoxide anions, and hydrogen peroxide [31]. However, it is still a subject of investigation as to which of these reactive oxygen species are directly involved in the damage to bacterial cells.
Catalyst deactivation is one of the drawbacks on the application photocatalytic degradation processes; deposition of photo insensitive species on the photocatalyst surface blocking its active sites. The economy of the photocatalytic process depends upon how many times a catalyst can be reused without losing its efficiency and the type of regeneration it requires. The SnO2 used catalyst was regenerated by treating with boiling distilled water and then by drying it in a hot air oven at a temperature of 100°C. The catalysts under consideration have not shown a significant decrease in their photoinduced and dark microbiocide efficiency when the antimicrobial experiments were repeated three times using the same sample.
SnO2 nanoparticles show much higher activity against E. coli than S.aureus. The higher deactivation efficiency in the case of Gram-negative bacteria as compared to Gram-positive ones was reported earlier [32, 33]. This difference can be attributed to the differences in the cell wall structure inherent in Gram-negative and Gram-positive bacteria. Gram positive and Gram negative bacteria have similar internal, but very different external structures. A Gram positive bacterium has a thick peptidoglycan layer that contains teichoic and lipoteichoic acids. A Gram negative bacterium has a thin peptidoglycan layer and an outer membrane that contains lipopolysaccharide, phospholipids, and proteins. This can lead to the conclusion that the bacteria photoinactivation rate is governed not only by cell wall thickness but also by the morphology of cell envelop and resistance of outer membrane to the reactive oxygen species produced at the photocatalyst surface. Figure 6 photographically shows two obtained results including the higher inactivation efficiency under UV illumination for both bacteria and much higher activity against E. coli than against S. aureus. In this study, the solvothermal method was employed to synthesize SnO2 nanoparticles with spherical morphology in the absence of templates or structure-directing agents under mild conditions. The nanoparticles used to in the inactivation efficiencies for two microorganisms under UV irradiation and dark conditions. It was found that SnO2 nanoparticles show much higher activity against E. coli than S.aureus.