1. Background
The L-arginine/nitric oxide (NO) pathway is a major defensive system in gastric mucosa because NO increases mucus generation and thickness and maintains adequate mucosal blood flow [1, 2]. Reduced NO synthase (NOS) activity predispose the elderly to nonsteroidal anti-inflammatory drug gastropathy [3]. It has been reported that using nitroglycerin, a drug that generates NO, is independently associated with a decreased risk of upper gasterointestinal bleeding, which is a result of both increase in blood flow of gastric mucosa and the inhibition of adherence of leukocyte to the endothelium [4].
NO is produced enzymatically from L-arginine by NOS and non-enzymatically from reduction of nitrite [5]. At least, three isoforms of NOS has been identified, i.e. endothelial NOS (eNOS), neural NOS (nNOS), and inducible NOS (iNOS) [5]. The existence of mitochondrial NOS (mtNOS) was reported in 1997 by Ghafourifar and Richter [6]. NOS-independent NO generation was first described in the stomach [1]; furthermore neural NOS have been found in 50% of enteric nervous system neurons and in parietal cells [7]. Non-enzymatic NO production from dietary nitrate/nitrite could be a potential blood and tissue reservoir of NO [8].
Oral ingestion of inorganic nitrate generates NO in gastric lumen [9]; in addition, enterosalivary circulation of ingested nitrate provides continuous production of NO in the gastric lumen [9]. Ingested nitrate is converted into nitrite in saliva, which, when swallowed, provides a protective mechanism against ingested pathogens by increasing bactericidal activity of gastric juice and could also act as a reservoir of NO [10]. The storage form of NO in tissues is limited [11] and nitrate, as a cytoprotective element in the diet [8], can restore NO homeostasis when NO production from NOS become dysfunctional [10]. L-arginine is a conditionally essential amino acid and it has been reported that L-arginine as a dietary supplement, could increase NO production [12, 13]. Despite low L-arginine doses being given as a food-additive for many years [14], some authors believe that considering the highly complex metabolism of L-arginine, exogenous arginine supplementation should be used with great caution or avoided [15].
NO levels in circulation may reflect changes in NO production by tissues [16]; however, it has not been completely clarified whether NOx status in tissues, is reflected in the plasma [17].
2. Objectives
Considering therapeutic approaches using nitrate/nitrite and L-arginine, the aim of this study was to determine the effect of nitrate and L-arginine administrations, as possible mechanisms for increased NO availability in tissues, on the serum, stomach, and liver concentrations of nitric oxide metabolites (nitrite+nitrate = NOx) in rats.
3. Materials and Methods
3.1. Animals and Study Protocol
In this interventional study, a total of 24 male Wistar rats (220 - 250 g and 14 weeks old) were maintained in standard conditions (temperature 22 ± 3˚C; relative humidity 50 ± 6%) with 12 hour light/dark cycles. All experiments were carried out in accordance with standards approved by the local ethics committee of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences. Animals, divided into 3 groups (n = 8 in each group), the control, nitrate, and L-arginine groups, had free access to standard rat chow (Pars Co., Tehran) and water during the study. Rats in the nitrate and L-arginine groups were administered sodium nitrate (500 mg/L) or L-arginine (2%) for one week in drinking water while the controls consumed tap water. At the end, after 12 - 14 hour fasting a blood sample was obtained for serum NOx measurement and stomach and liver samples for NOx measurement were prepared as previously described [18]; in brief, animals were anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and after thoracotomy, organs were flushed free of blood, using EDTA/N-ethylmaleimide solution (10/2.5 mmol/L) freshly prepared in phosphate buffer saline perfused through the left ventricle for 2 minute while blood drained through the right auricle. Stomach and liver were removed, weighed and homogenized in ice-cold perfusion solution by a homogenizer (Miccra D-1, Germany) and sonicated with a Microson XL 2000 sonicator (USA) while immersed in an ice-water bath.
3.2. Serum and Tissue NOx Measurement
Serum and tissue NOx concentrations were measured by the Griess reaction [18]. In brief, serum samples were deproteinized by zinc sulfate (15 mg/mL), and centrifuged at 10,000 g for 10 minute; tissue homogenates were first centrifuged at 15,000 g for 20 minute, then zinc sulfate (15 mg/mL) was added and after one minute shaking, samples were recentrifuged at 15,000 g for 20 minute. For both serum and tissue samples, a 100 µL of the supernatant was transferred to a microplate well, and 100 µL vanadium (III) chloride (8 mg/mL) was added to each well to reduce nitrate to nitrite, as the Griess reaction detects only nitrite. Griess reagents [50 µL sulfanilamide (2%) and 50 µL N-ethylenediamine dihydrochloride (0.1%)] were then added and samples were incubated for 30 minute at 37˚C; absorbance was read at 540 nm using the ELISA reader (Sunrise, Tecan, Austria). NOx concentration was determined from the linear standard curve established by 0 - 150 µmol sodium nitrate. Inter- and intra-assay coefficients of variation were 5.2% and 4.4% respectively. The sensitivity of the assay was 2.0 µmol/L and its recovery was 93 ± 1.5%. The protein content of the homogenates was determined by the Bradford method [19] and bovine serum albumin (BSA) was used as a standard; tissue NOx levels were expressed as nmol/mg protein.
SPSS-20 was used for statistical analyses. Because of the skewed distribution of NOx values, non-parametric statistics were used and data were presented as median (interquartile range). Kruskal-Wallis one-way ANOVA was used to compare the effects of sodium nitrate and L-arginine administration in different groups and Mann-Whitney U test was used for pairwise comparison. Spearman correlation coefficient was calculated between serum and tissue NOx levels. Two-sided P values < 0.05 were considered statistically significant.
4. Results
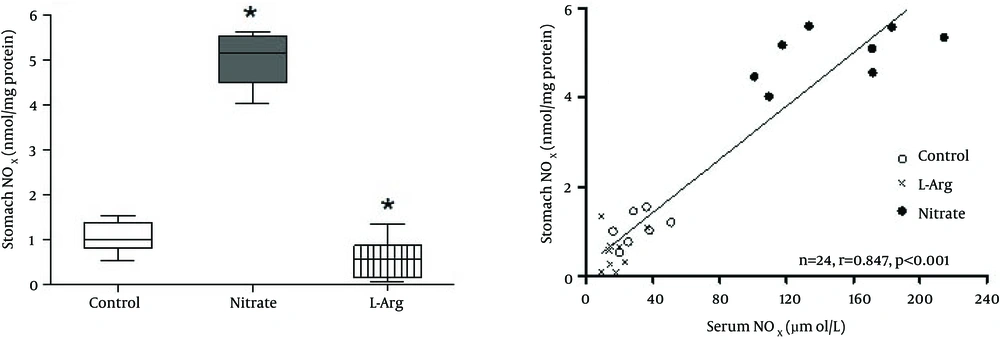
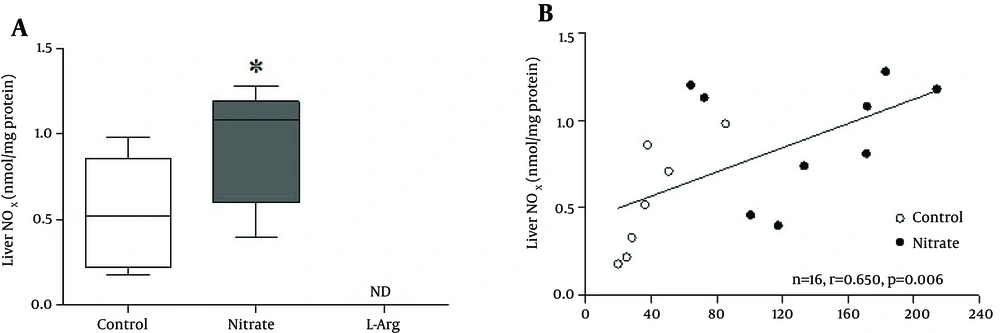
In the control group, median (interquartile range) stomach NOx was 1.02 (0.81 - 1.34) μmol/L; nitrate administration, significantly (P < 0.001) increased 5.16 (4.50 - 5.53) μmol/L and L-arginine administration significantly (P < 0.05) decreased 0.57 (0.17 - 0.87) μmol/L stomach NOx levels (Figure 1 A). Liver NOx in the control group was 0.52 (0.22 - 0.86) μmol/L; nitrate administration, significantly (P < 0.05) increased 1.08 (0.60 - 1.19) μmol/L liver NOx levels while L-arginine administration decreased it to non-detectable levels (Figure 2 A). Median (interquartile range) serum NOx concentrations in the control group 28.2 (19.6 - 37.8) μmol/L differed significantly (P < 0.05) from those of the nitrate 152.4 (111.4 - 180.2) μmol/L, and L-arginine 14.5 (11.2 - 21.5) μmol/L groups.
Spearman correlations between serum and tissue NOx are shown in Figures 1 B and 2B. Positive correlations were observed between serum and the stomach (r = 0.847, P < 0.001) and liver (r = 0.650, P = 0.006) NOx levels.
A, Box plots showing the effects of sodium nitrate and L-arginine administration on NOx levels of the stomach. Median (interquartile range) stomach NOx was 1.02 (0.81 - 1.34) μmol/L in the control group; nitrate administration significantly (P < 0.001) increased 5.16 (4.50 - 5.53) μmol/L and L-arginine administration significantly (P < 0.05) decreased 0.57 (0.17 - 0.87) μmol/L stomach NOx levels; B, Correlation between serum and stomach NOx content. * Significant difference compared to control group.
A, Box plots showing the effects of sodium nitrate and L-arginine administration on NOx levels of the liver. Liver NOx in the control group was 0.52 (0.22 - 0.86) μmol/L; nitrate administration significantly (P < 0.05) increased 1.08 (0.60 - 1.19) μmol/L liver NOx levels while L-arginine administration decreased it to non-detectable levels; B, Correlation between serum and liver NOx content. * Significant difference compared to control group; ND, non-detectable.
5. Discussion
The results of this study indicated a 4.1-fold and 1.1-fold increases in stomach and liver NOx contents respectively of rat following one week oral nitrate administration, demonstrating the effect of dietary nitrate administration on systemic NO metabolites [18, 20] and tissue nitrite [18-21] levels. In line with our results, Jansson et al. [1] have reported 10.7 folds increase in nitrate levels of stomach following one-week administration of sodium nitrate of 1 mmol/kg in rats, a dose approximately twice that which we used in the current study (500 mg/L or 0.6 mmol/kg). Raat et al. [8] have reported 1.5-fold and 2.3-fold increases in stomach nitrate content following one-week administration of 300 mg/L and 1,500 mg/L sodium nitrite respectively. Nitrate is considered as a prodrug of nitrite [22] and similar to our results Duranski et al. [23] have reported that nitrite treatment increases liver nitrite levels in mice.
The changes we observed in stomach and liver NOx contents following nitrate administration were different; it has been reported that differences in nitrite concentrations between tissues may reflect the degree of NOS activity and the oxidation pathways of NO [24]; however, increased NOx content of the stomach following nitrate administration may also be attributed in part to nitrate absorption from the stomach [25].
In the current study, we found relatively high correlations between serum concentrations and gastric and liver NOx levels, a finding in line with a previous report that nitrate concentration of the blood is a major determinant of NOx levels of the rest of the body [26]. Close correlation between plasma and tissue nitrite after nitrite administration has been previously reported [24]. Similar to our results, Raat et al. [8] have reported a direct correlation between plasma and liver nitrite concentrations; some authors have suggested that high correlation between serum and some tissue NOx indicates non-specific accumulation of NOx in these organs [27] while others suggest that anion transporters aid regulated and tissue-specific transport of nitrite across cell membranes [24, 28].
Recent findings suggest that nitrate/nitrite could be considered as potential therapeutic agents [29, 30]. Following oral nitrate intake, large amounts of NO is produced in stomach, amounts greater than that required for vasodilation; the excess amount can contribute to host defense and in gastric physiology [31, 32]. One-week nitrate therapy has prevented gastric injury induced by diclofenac in rats, which may be due to increased intragastric NO formation and stimulation of mucus formation [1]. On the other hand, cancer, in particular stomach cancer, was a concern of nitrate/nitrite consumption [10, 29]. In our study, according to food and water intake measurements, rats received 13 and 51 mg/kg/day nitrate for one week in the control and nitrate groups respectively. It has been reported that sodium nitrite of 130 mg/kg in male rats for 2 years is not carcinogen [10]. Although still in doubt, it has been recently reported that old hypothesis of association between (stomach) cancer and ingested nitrate/nitrite is not supported by new data and there is no evidence implicating nitrate/nitrite as an animal or human carcinogen [10].
In the current study, L-arginine administration decreased levels of stomach NOx by 44% and those of liver NOx to non-detectable values. In line with our results, Ohta and Nishida [2] have reported that administration of L-arginine could prevent stress-induced increases in the gastric mucosa NOx levels in rats. L-arginine increases arginase activity, which could decrease NO production by NOS [18] via reducing substrate availability [33]. In addition, decarboxylation of L-arginine by the arginine decarboxylase produces agmatine [34], which is a competitive inhibitor of the NOS isoenzymes [35] and could inhibit all isoforms of NOS and NO production [36, 37]. While there are several reports of the protective effect of L-arginine administration against development of gastric mucosal lesion [2], it has recently been reported that L-arginine metabolism could impair antimicrobial NO synthesis in stomach and cause H. pylori induced DNA damage [38]. In addition, it seems that L-arginine does not stimulate NO production in vitro unless during L-arginine deficiency; some of L-arginine actions in vivo, previously attributed to increase NO production, may be due to other mechanisms including increase in insulin secretion [39].
In conclusion, the results of this study indicate that nitrate and L-arginine administration had opposite effects on the NOx levels in the stomach and liver of normal rats. In addition, direct correlations were observed between serum and the tissues NOx levels, findings which may be important considering the fast accumulating evidence on the protective roles of dietary nitrate and nitrite.