1. Background
Although opium is frequently used for the synthesis of some applied medications such as morphine, noscapine, papaverine, and codeine, its deleterious effects on vital organs and biomarkers have been reported. Some studies could examine liver function abnormalities following opium use and misuse that liver destructive and metabolic changes identified in the opium addicted patients [1]. Opium use cause liver damage, increased the activity of liver enzymes including aspartate aminotransferase (AST), alanine aminotransferase (ALT) and can lead to the sharp increase of thymol test [2]. These destructive effects of opium have been also observed in biliary systems so that a significant increase in the range of the common bile duct diameter in comparison with normal bile ducts was shown following chronic opium addiction [3]. Besides, it has been suggested that opioid neurotransmission is increased in patients with liver disease, and therefore, administration of opioid antagonists decreases liver injury in animal models with acute biliary obstruction [4, 5]. Moreover, the current data suggest that regular injection of opioids may lead to decreased hepatic glutathione levels [6]. Glutathione is used by every cell in the body to reduce free radicals and prevent damages to the immune system.
On the other hand, safely increasing glutathione levels shows great promise as a potential option for many inflammatory conditions and thus opium use can change these conditions and result in impaired liver immune system [7].
In parallel, interesting studies published focused specifically on the effect of cigarette smoking on the liver damage and inflammation. It has been demonstrated that current and former smokers has more inflammation and scarring of liver than nonsmokers [8]. The numerous toxins found in cigarette tobacco lead to chronic inflammation and scarring in the liver, which in turn, increases the risk for liver damage including diseases such as liver cancer and fibrosis. In addition, smoking affects the way the liver processes alcohol and medications, which can increase the risk for alcoholism as well as your overall drug and alcohol tolerance levels [9]. However, no study is available with respect to comparing effects of cigarette smoking and opium use on liver function.
2. Objectives
The present study came to address the degree of liver inflammation following inhaled opium use and compare it with the severity of liver inflammation in the animal group receiving cigarette smoking.
3. Materials and Methods
In this experimental study, 40 Syrian golden hamsters (weighting 90 - 110 g) were entered into the study. Animals were kept at 22 ± 1°C and normal photo period (12 hour dark/12 hour light) and had freely accessed to food and water. All animals’ procedures were in accordance to guide for the care and use of laboratory animals (NIH US publication no. 85-23 revised 1985). The experimental protocol has been approved by the Animal Experimentation Ethic Committee of the Kerman University of Medical Sciences. The hamsters were randomly divided into 5 groups: control group (did not receive any experiment agents), group A (receiving inhaled opium), group B (receiving cigarette smoke), group C (receiving inhaled opium with double dosage of the second group), and group D (receiving inhaled opium and cigarette smoke concurrently). In detail, group A were transferred into a closed room (2.0 × 1.5 × 1.5 m3) twice a day, 6 hours apart and inhaled opium (derived from 250 mg opium for each test) was released in the room by a special device made at the Physiology Research Center of our university. To ensure the quality of opium effectiveness, naloxone was injected subcutaneously (s.c.) at dose 4 mg/kg and assessed the withdrawal syndromes of morphine relief. For group B, the samples were transferred to into a similar closed room and exposed to cigarette smoke (5 cigarettes four times a day every 30 minute, for 5 days a week and totally for 4 weeks). Group C underwent the same protocol at group A, but with double dosage of opium. Group D was exposed to inhaled opium and cigarette smoke concurrently.
For determining purity of opium samples, GC-mass spectrometry analysis was used. The samples were dissolved in a suitable solvent and injected into the GC-mass spectrometry system. Atomic absorption spectro-metry was also used to determine the amount of impurities of opium samples and repeated for at least three times to minimize measurement error. Analysis of this opium (Table 1) showed 11.251% codeine, 21.299% morphine, 10.498% papaverine and 56.952% alpha-epoxy-3-methoxy-17-methyl-7alpha- (4-phenyl-1, 3-butadienyl)-6 beta, 7 beta (oxymethylene) morphinan (Table 1).
| Peak | TR | Compound | Area | Percent | RI |
|---|---|---|---|---|---|
| 63.183 | Codeine | 36889277 | 11.251 | 2076 | |
| 65.665 | Morphine | 69836048 | 21.299 | 2134 | |
| 75.422 | Papaverine | 34421168 | 10.498 | 2360 | |
| 77.956 | 4, 5 alpha-epoxy-3-methoxy-17-methyl-7 alpha- (4-phenyl-1, 3-butadienyl)-6 beta, 7 beta-(Oxymethylene) morphinan | 186732521 | 56.952 | 2419 |
After 4 weeks of experiments, the hamsters were anesthetized with 50 mg/kg ketamine, and then, were killed by 3 mg potassium chloride injected into the heart. The liver of hamsters was removed. For paraffin embedding, tissue samples were fixed in 10% buffered formaldehyde for 24 hours at room temperature. Sections of 4 µm thickness were mounted and stained in Haematoxylin-Eosin. Finally, a random number was assigned to each Hematoxylin and Eosin-stained lung section from the treatment groups. A pathologist blinded to the random numbers evaluated the slides for the degree of inflammation using a Zeiss photomicroscope. The degree of liver inflammation was determined by three scoring approaches (Table 2): in first scoring method, the liver sections per mouse were scored in a blinded manner by a pathologist according to the scoring parameters outlined below:
0) Normal; no abnormalities observed; 1) Minimal; few enlarged hepatocytes, mitotic cells may be present, no microsteatosis; 2) Mild; minimal hepatocyte enlargement around the central vein, scattered lymphocytic infiltration, mitotic cells may be present; 3) Moderate; hepatocellular enlargement around the central veins, pyknotic nuclei, scattered foci of hepatocyte dropout, lymphocytic infiltration, mitotic cells may be present; 4) Severe; extensive hepatocellular enlargement of central vein regions, pyknotic nuclei, cellular necrosis, granular cytoplasm, widespread hepatocellular dropout, lymphocytic infiltration, mitotic cells present, portal triad regions primarily unaffected and 5) Complete involvement-widespread hepatocellular necrosis and hemorrhage in central regions. Cells in portal triads are less affected, but do show cytoplasmic granulation and an increase in size [10]. Results were presented as Mean ± SD for quantitative variables and were summarized by absolute frequencies and percentages for categorical variables. Categorical variables were compared using χ2 test or Fisher’s exact test when more than 20% of cells with expected count of less than 5 were observed. Quantitative variables were compared using ANOVA test. For the statistical analysis, the statistical software SPSS-19 (SPSS Inc., Chicago, IL) was used. P values ≤ 0.05 were considered statistically significant.
| Score | Knodell/Original HAI | Ishak/Modified HAI | METAVIR |
|---|---|---|---|
| No portal inflammation | None | Absent | |
| Mild (sprinkling of inflammatory cell in less than 1/3 of portal tracts) | Mild, some or all portal areas | Presence of mononuclear aggregates in some portal tracts | |
| Moderate, some or all portal areas | Mononuclear aggregates in all portal tracts | ||
| Moderate (increased inflammation in 1/3 to 2/3 of portal tracts | Moderate/marked, all portal areas | Large and dense mononuclear aggregates in all portal tracts | |
| Marked (dense packing of inflammatory cells in more than 2/3 of portal tracts) | Marked, all portal areas |
4. Results
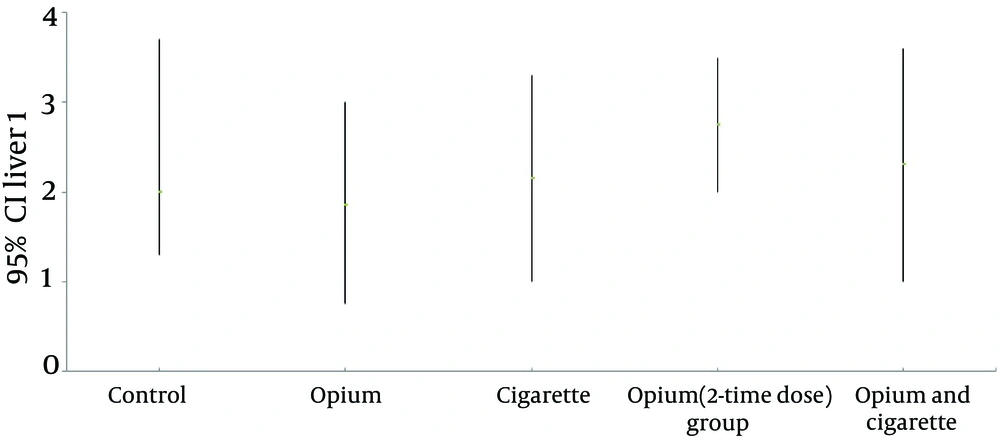
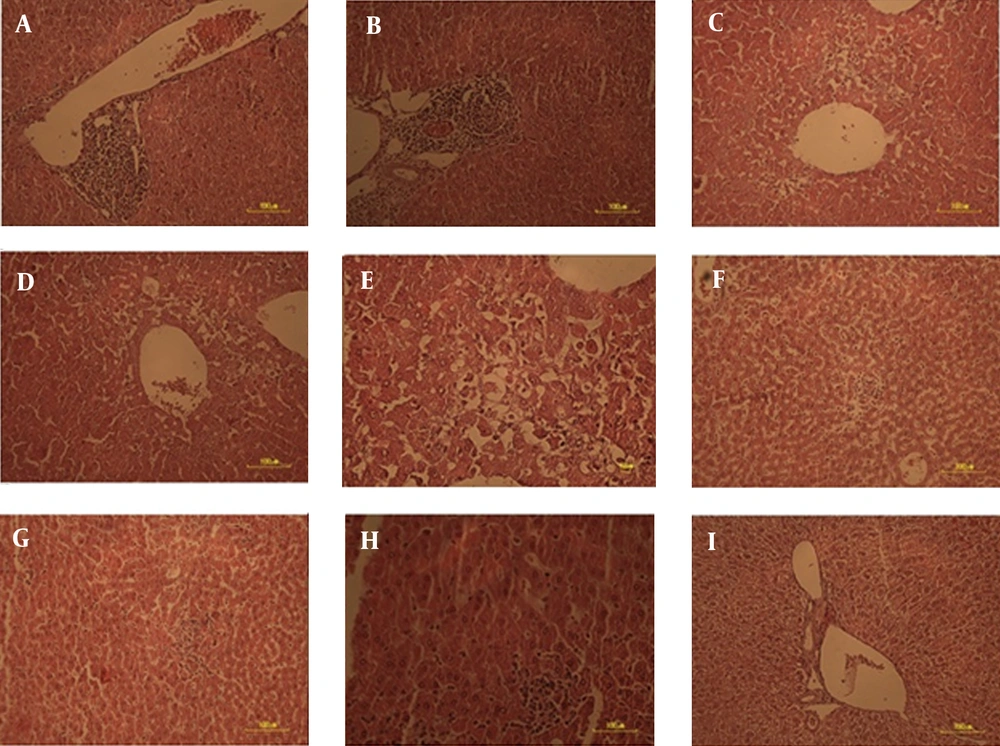
According to the first method scoring inflammation, there was no significant difference in the degree of inflammation activity across the intervention and control groups (Table 3). The mean inflammation score was also similar between the 5 groups (Figure 1). Histological interpretation of the liver samples also showed similar mean Knodell/original HAI score Ishak/modified HAI score and METAVIR scores across the 5 groups (Table 4, Figure 2 A - I).
| Inflammation Score | Control | Opium | Cigarette | Opium (2-Time Dose) | Opium and Cigarette | P Value |
|---|---|---|---|---|---|---|
| 1 (12.5) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 0.327 | |
| 0 (0.0) | 3 (37.5) | 4 (50.0) | 0 (0.0) | 2 (25.0) | ||
| 3 (37.5) | 1 (12.5) | 0 (0.0) | 3 (37.5) | 0 (0.0) | ||
| 1 (12.5) | 2 (25.0) | 2 (25.0) | 3 (37.5) | 3 (37.5) | ||
| 3 (37.5) | 1 (12.5) | 2 (25.0) | 2 (25.0) | 2 (25.0) |
a Values are presented as No. (%).
| Type of Inflammation | Score | P Value |
|---|---|---|
| 0.591 | ||
| Control | 2.13 ± 1.25 | |
| Opium | 1.38 ± 1.06 | |
| Cigarette | 1.75 ± 1.04 | |
| Opiume (double dose) | 1.50 ± 0.93 | |
| Opium and cigarette | 1.38 ± 1.06 | |
| 0.762 | ||
| Control | 1.88 ± 1.13 | |
| Opium | 1.50 ± 0.93 | |
| Cigarette | 1.75 ± 0.89 | |
| Opiume (double dose) | 1.38 ± 0.74 | |
| Opium and cigarette | 1.88 ± 0.99 | |
| 0.668 | ||
| Control | 1.75 ± 1.17 | |
| Opium | 1.25 ± 0.89 | |
| Cigarette | 1.63 ± 0.92 | |
| Opiume (double dose) | 1.25 ± 0.46 | |
| Opium and cigarette | 1.25 ± 0.89 |
a Values are presented as mean ± SD.
A) Normal; no abnormalities observed; B) Minimal; few enlarged hepatocytes, mitotic cells may be present, no microsteatosis; C) Mild; minimal hepatocyte enlargement around the central vein, scattered lymphocytic infiltration, mitotic cells may be present; D) Moderate; hepatocellular enlargement around the central veins, pyknotic nuclei, scattered foci of hepatocyte dropout, lymphocytic infiltration, mitotic cells may bepresent; E) Severe; extensive hepatocellular enlargement of central vein regions, pyknotic nuclei, cellular necrosis, granular cytoplasm, widespread hepatocellular dropout, lymphocytic infiltration, mitotic cells present, portal triad regions primarily unaffected, F) Complete involvement-widespread hepatocellular necrosis, G) Haemorrhage in central regions, H) Cells in portal triads are less affected and I) show cytoplasmic granulation and an increase in size.
5. Discussion
Based on the results using three scoring methods, no significant differences were revealed in liver inflammation scores across the groups receiving tobacco smoke or inhaled opium. Also, the degree of appeared liver inflammation was comparable between the different interventional groups and the control which not received cigarette smoking or opium. Regarding impact of smoking on liver diseases, it has been demonstrated that the exposure to environmental tobacco smoke especially on a hypercholesterolemic background increases liver injury through oxidative/nitrative stress, hypoxia, and mitochondrial damage [11, 12].
Cigarette smoke damages the microvasculature via endothelial cell dysfunction and smooth muscle cell proliferation leading to impair NO signaling and tissue hypoxia [13]. Recent epidemiologic studies also suggest that cigarette smoke exposure accelerates a number of chronic liver diseases including hepatitis C and primary biliary cirrhosis, and increases the risk for hepatocellular carcinoma [14-18]. With respect to the influence of cigarette smoking on hepatic inflammation, it has been also demonstrated that the smoking yields chemical substances with cytotoxic potential which increase necro-inflammation and fibrosis. In addition, smoking can increase the production of pro-inflammatory cytokines such as IL-1, IL-6 and TNF-α that would be involved in liver cell injury. Smoking affects both cell-mediated and humoral immune responses by blocking lymphocyte proliferation and inducing apoptosis of lymphocytes [14]. Smoking also increases serum and hepatic iron which induce oxidative stress and lipid peroxidation that lead to activation of stellate cells and development of fibrosis [19]. The similarity between the effects of opium use and smoking in our study hypothesized that each of above mechanisms might be involved in the mechanisms of the effects of opium use on appearance and severity of hepatic inflammation. However, these mechanisms did not evaluate previously and should be taken into consideration in further studies. The findings about the triggering or inhibitory effects of opiates on inflammation systems are now conflicting. Although some studies emphasized anti-inflammatory effects of some opiates such as morphine [20], some others showed that the dual effect of opium on inflammation might be dependant to the type of the opioid receptors in the especial organs [21], that µ opioid receptor activation prevents acute hepatic inflammation and cell death and activation of the δ-opioid receptor inhibits serum deprivation induced apoptosis of human liver cells via the activation of PKC (protein kinase C) and the mitochondrial pathway [22, 23], while some studies showed that administration of opioid receptors antagonists attenuate liver fibrosis [24]. Thus, impact of opium on liver inflammation systems should be assessed considering the types of activated opiate receptors in liver tissue. In summary, the inflammatory response to cigarette smoking seems to be similar to the use of inhaled opium; however similarity of the inflammation degree between inhaled opium users and control experiments should be taken into consideration.