1. Background
Malaria and HIV-1 are the 2 most prevalent infections in developing countries, particularly in sub-Saharan Africa (1). An estimated 36.9 million people are living with HIV, and 1.8 million are newly infected across the globe (2). Malaria is the fourth leading cause of death of children between 0 - 5 years, as well as pregnant women, in developing countries (3, 4). An estimated 85 million pregnancies were reported in the areas of endemic Plasmodium falciparum infection (5). Sub-Saharan Africa bears the brunt of this epidemic, where 25.7 million individuals were infected (2). The immunosuppression caused by HIV infection poses a negative effect on the immune response against P. falciparum, leading to distinct disease outcomes in HIV infection (6).
Providing the overlap of both infections’ geographic distribution and resultant rates of co-infection, interactions between malaria and HIV pose major public health concerns (7). While both malaria and HIV can cause disease and death, infection with one can exacerbate infection with the other and make clinical management more challenging (8). When pregnant women are infected with both HIV/AIDS and malaria, the implications are more severe (8). These infections interact with one another synergistically and bidirectionally; this is because HIV infection increases the likelihood and severity of malaria infection; accordingly, increased parasite burdens could lead to higher rates of malaria transmission (9). HIV infection appears to reduce pregnant women’s malaria immunity because HIV-infected pregnant women show more frequent and higher-density parasitemia than those without HIV (10, 11).
Cytokines are key players in coordinating immune responses between cells as they bind to a variety of receptors to induce cell-specific immune responses (12). Like other human diseases, malaria is caused by the production of pro-inflammatory cytokines, stimulating the production of additional cytokines and resulting in tissue pathology (13). The anti-inflammatory cytokines, particularly IL-4 and IL-10, specifically act by inhibiting the production of pro-inflammatory cytokines (14). During successful pregnancies, fetal trophoblasts and maternal leukocytes produce predominantly T helper 2 (Th2) type cytokines to prevent initiation of inflammatory and cytotoxic type responses, which might damage the integrity of the materno-fetal placental barrier (12). HIV infection could impair immunity to malaria as it alters the cytokine profile (15, 16). The pro- and anti-inflammatory cytokines have been found to be significantly elevated in the peripheral blood and intervillous spaces of the placentas of malaria-infected women. The production of these cytokines is responsible for the resultant Th1: Th2 imbalance observed in P. falciparum–infected placentas (17, 18). There is a dearth of information on the immunological mechanism occurring in pregnant women co-infected with HIV and P. falciparum in Benin City.
2. Objectives
This study was conducted to determine some pro- and anti-inflammatory cytokines among pregnant women co-infected with HIV and P. falciparum in Edo State, Nigeria.
3. Methods
3.1. Study Area
This study was conducted at Central Hospital, Benin City, Edo State. Central Hospital is a secondary health institution with referral status in the management of HIV infection. In addition, the obstetrics and gynecology clinic receive referred cases from primary health care clinics and maternity homes in the state. The ancient Benin City is the capital of Edo State, with an estimated population of 1.496 million (19).
3.2. Study Population
This study was conducted between March 2019 and January 2020. A total of 200 participants were enrolled, consisting of 150 pregnant women co-infected with HIV and P. falciparum and 50 non-pregnant women (as controls) who were neither infected with HIV nor P. falciparum. Pregnant women with confirmed HIV and P. falciparum and those that consented to participate were included in this study. Pregnant women without HIV and P. falciparum and those that refused consent were excluded from this study. A structured questionnaire focusing on biodata was administered to each participant before specimen collection. Informed consent was obtained from each participant before enrolment. The protocol for this study was approved by the Ethics and Research Committee of the Ministry of Health, Benin City, Edo State (code: HA577/VOL.2/192).
3.3. Sample Size
The sample size was calculated using the formula
3.4. Specimen Collection
About 5 mL of venous blood was collected from participants, of which 2.5 mL was dispensed into ethylene diamine tetra acetic acid and mixed, while the balance, 2.5 mL, was dispensed into plain containers, separated and serum was obtained for cytokines assay.
3.5. Processing of Specimen
Malaria diagnosis was conducted by preparing thick and thin blood films from the anticoagulated blood sample, stained in 10% Giemsa solution, and examined microscopically (20). CD4 T-lymphocyte count was determined using a flow cytometer (Partec, GmbH, Germany). Briefly, 20 mL of CD4 PE antibody was dispensed into a Partec tube, and 20 mL of well-mixed whole EDTA blood was added, mixed gently, and incubated in the dark for 15 minutes at room temperature. Approximately 800 µL of CD4 buffer was added to the mixture and mixed gently while plugged into the counter for counting.
Human IL-2, IL-10, and IFN-γ were assayed using the respective cytokines commercial ELISA kits (MELSIN Medical Co Limited, Jillin, China). Dilutions of the standard were prepared with the following concentrations 240 ng/L, 160 ng/L, 80 ng/L, 40 ng/L, and 20 ng/L. About 50 µL of standards were pipetted into the wells, and 10 µL of test serum was added to each sample well. Forty microliters of sample diluent was added to each sample well. A sample blank was included in the run. Fifty microliters of HRP-conjugate reagent was added to each except that of blank, covered with an adhesive tape, and incubated for 30 minutes at 37°C. The mixture was washed 4 times. Approximately 50 µL of chromogen solution A and 50 µL of chromogen solution B were added to each well. The preparations were mixed and incubated for 10 minutes at 37°C. About 50 µL of stop solution was added to each well and read at 450 nm wavelength within 15 minutes. A standard curve of optical density against the concentrations of standards was plotted, and the concentrations of IL-2, IL-10, and IFN-γ were determined, respectively.
3.6. Statistical Analysis
Data were analyzed using the Student t-test and analysis of variance (ANOVA). Turkey test was used as a post hoc test for if ANOVA was significant. The statistical software used in the analyses was INSTAT® (Graph Pad Software Inc, La Jolla, CA, USA).
4. Results
IL-2, IL-10, and IFN-γ values were lower in pregnant women with HIV than in their HIV-negative counterparts, with only values for IL-10 failing to reach statistical significance (Table 1). Among HIV-infected pregnant women, IL-2 (P = 0.0070), IL-10 (P = 0.0179), and IFN-γ (P = 0.1564) values were lower in primiparous women compared with multiparous women (Table 2). Married pregnant women with HIV had significantly higher IL-2 (P = 0.0085) and IFN-γ (P = 0.0332) levels compared with single women, while marital status did not affect the IL-10 level of pregnant women with HIV (Table 3). Only IL-2 levels of the HIV-infected pregnant women increased significantly (P = 0.0012) with increasing trimester (Table 4).
| Parameters | HIV (n = 150) | Control (n = 50) | P-Value |
|---|---|---|---|
| IL-2 (pg/mL) | 517.42 ± 30.70 | 804.60 ± 37.79 | < 0.0001 |
| IL-10 (pg/mL) | 148.74 ± 10.31 | 170.27 ± 16.29 | 0.2875 |
| IFN-γ (pg/mL) | 179.42 ± 6.93 | 221.77 ± 14.45 | 0.0101 |
Relationship Between Tested Cytokines and HIV Status Among Pregnant Women
| Parameters | Primiparous (n = 44) | Multiparous (n = 106) | P-Value |
|---|---|---|---|
| IL-2 (pg/mL) | 392.85 ± 53.23 | 575.58 ± 36.92 | 0.0070 |
| IL-10 (pg/mL) | 108.59 ± 17.13 | 161.79 ± 12.42 | 0.0179 |
| IFN-γ (pg/mL) | 164.46 ± 12.66 | 186.54 ± 8.49 | 0.1564 |
Relationship Between Tested Cytokines and Parity Among HIV-Infected Pregnant Women
Association Between Tested Cytokines and Marital Status Among HIV-Infected Pregnant Women
| Trimester | IL-2 (pg/mL) a | IL-10 (pg/mL) | IFN-γ (pg/mL) |
|---|---|---|---|
| First (n = 49) | 358.32 ± 40.75 | 129.94 ± 18.83 | 166.19 ± 13.55 |
| Second (n = 86) | 571.32 ± 42.86 | 152.73 ± 13.06 | 185.41 ± 9.15 |
| Third (n = 15) | 680.76 ± 103.66 | 188.71 ± 39.89 | 196.10 ± 20.45 |
| P-value | 0.0012 | 0.2762 | 0.3622 |
Relationship Between Tested Cytokines and Gestational Age Among HIV-Infected Pregnant Women
The presence of malaria infection did not significantly alter the levels of the tested cytokines among the HIV-infected pregnant women (P = 0.5131 (IL-2); P = 0.3274 (IL-10); P = 0.4651 (IFN-γ). The tested cytokines levels were lower in those with malaria compared with those without malaria among HIV-infected pregnant women. Conversely, among non-pregnant and non-HIV subjects, the presence of malaria resulted in higher levels of the tested cytokines (Table 5).
| Parameters | Positive Malaria | Negative Malaria | P-Value |
|---|---|---|---|
| HIV patients | N = 18 | N = 132 | |
| IL-2 (pg/mL) | 462.81 ± 85.02 | 524.87± 32.97 | 0.5131 |
| IL-10 (pg/mL) | 121.29 ± 25.84 | 152.48 ± 11.17 | 0.3274 |
| IFN-γ (pg/mL) | 105.66 ± 15.73 | 181.30 ± 7.58 | 0.4651 |
| Controls | N = 6 | N = 44 | |
| IL-2 (pg/mL) | 888.43 ± 72.25 | 793.17 ± 41.73 | 0.4184 |
| IL-10 (pg/mL) | 307.62 ± 43.11 | 151.54 ±15.70 | 0.0012 |
| IFN-γ (pg/mL) | 265.88 ± 51.33 | 215.76 ± 14.89 | 0.2641 |
Association Between Tested Cytokines and Malaria Infection Among HIV-Infected Pregnant Women
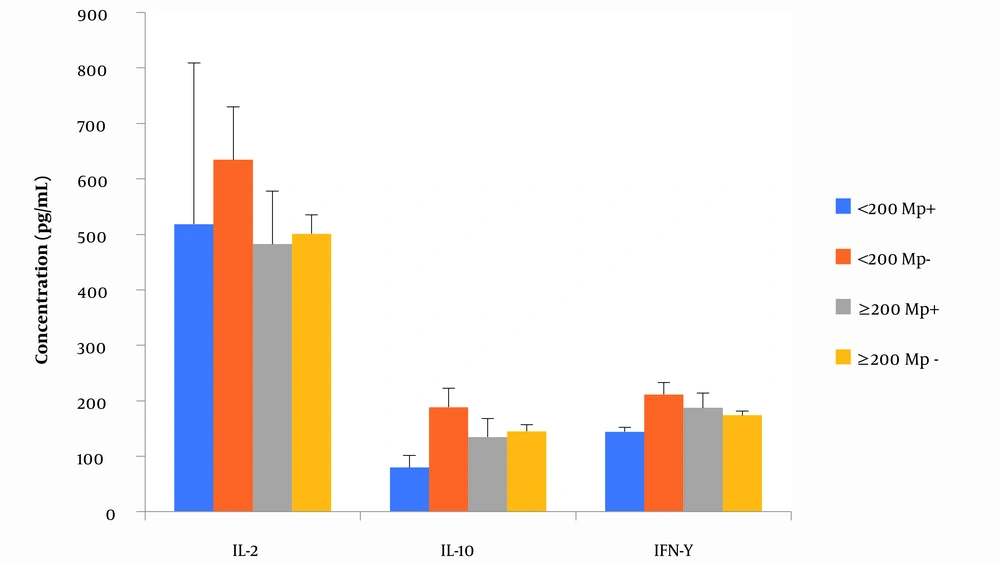
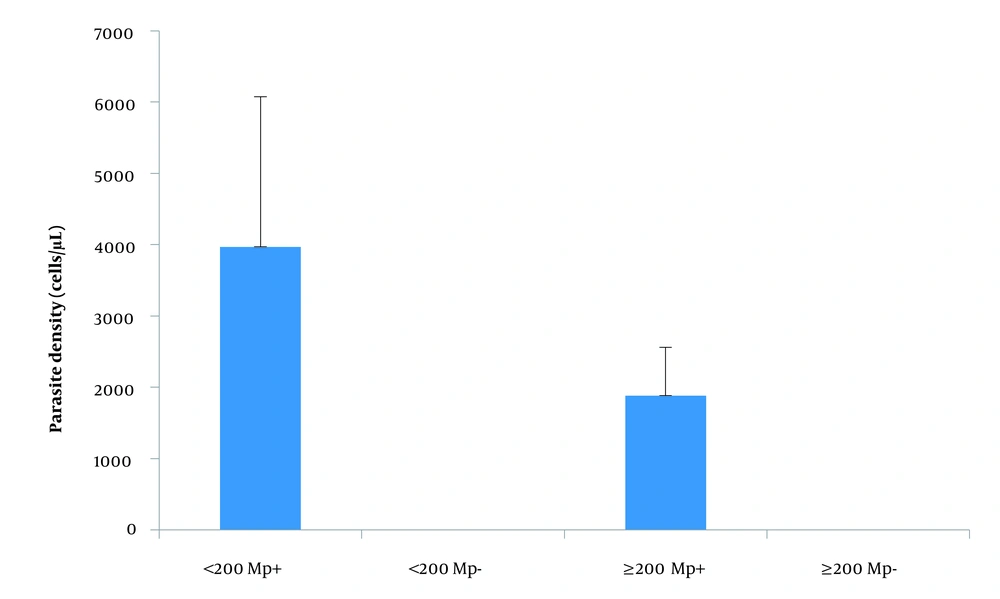
Neither immunosuppression (as measured by CD4 count < 200 cells/µL) nor malaria infection altered the levels of IL-2, IL-10, and IFN-γ (Figure 1) and parasite density (Figure 2) among pregnant women with HIV. Generally, there was no significant correlation between CD4 count, parasite density, and the tested cytokines (data not shown).
5. Discussion
Malaria and HIV are 2 of the most common and important health problems in sub-Saharan African countries, and pregnant women are particularly a vulnerable group (21). In pregnancy, the body undergoes modulation of pro-inflammation responses to ensure fetal survival (22). These adjustments may decrease maternal immune defenses and promote infections (17). HIV infection may impair immunity to malaria by altering cytokine profile (22). A number of factors have been reported to influence cytokine levels in an individual (23). Some pregnancy-associated malaria effects, such as maternal anemia, low birth weight, preterm delivery, and increase-infant and maternal mortality, have been attributed to overexpression of cytokines, as high cytokine levels suppress the cell-mediated immune response (24-26). Against the background of the paucity of data on cytokine levels among pregnant women co-infected with HIV and malaria, this study was conducted.
In this study, the levels of IL-2, IL-10, and IFN-γ were lower in pregnant HIV women compared with non-pregnant and non-HIV counterparts. Among non-pregnant HIV-1–infected women, decreased levels of IL-2 have been reported, associated with impairment of CD4 leucocyte count (27, 28). IL-2 is primarily produced by CD4 lymphocytes, and it promotes the proliferation of T- and B-lymphocytes, as well as induces the secretions of other cytokines such as IFN-γ, IL-4, and tumor necrosis factor α (TNF-α) (28-30). Sutton et al. (28) suggested that pregnancy might downregulate the production of IL-2. This may explain the reason for our finding.
It has generally been reported that primigravidae have a higher prevalence of malaria than multigravidae (31), meaning that primigravidae are more susceptible to malaria. Reduced IL-2 associated with pregnancy (28) and HIV-1 infection (27, 28) may be more pronounced in primigravidae. This will lead to lower production of other cytokines (28). This may explain the lower cytokine levels of primigravidae compared with multigravidae.
Stress from marital conflicts, as well as hostile interaction during marital conflicts, has been reported to affect pro-inflammatory cytokine levels (32, 33). In this study, IL-2 and IFN-γ were significantly higher in married HIV-pregnant women compared with their counterparts. IL-10 is an anti-inflammatory cytokine (14). Marital status did not affect the tested cytokines levels. Therefore, the finding of this study is in agreement with the observations of Kiecolt-Glaser et al. (32) and Graham et al. (33).
IL-2, IL-10, and IFN-γ levels increased with increasing trimester; however, this increase was only significant for IL-2 (P = 0.0012), where IL-2 levels in the first trimester of pregnancy were significantly lower compared to the second and third trimesters (P < 0.01 each). As earlier noted, pregnancy suppresses IL-2 production (28). It is plausible that as gestational age (trimester) increases, the suppression of IL-2 eases off gradually, which may be responsible for an increase in the second and third trimesters. Further studies on the correlation between IL-2 and gestational age may be needed to verify this finding.
Among HIV pregnant women with malaria, the levels of IL-2, IL-10, and IFN-γ were insignificantly lower (P = 0.5131 [IL-2]; P = 0.3274 [IL-10]; P = 0.4651 [IFN-γ]) than their counterparts. This finding agrees with the report of Sutton et al. (28) in relation to IL-2 and IFN-γ. Among non-pregnant and non-HIV infected women, IL-2, IL-10, and IFN-γ levels were higher in subjects with malaria compared with those without malaria, with that for IL-10 only reaching statistical significance (P = 0.0012). A similar picture was reported for IL-10 by Sutton et al. (28); however, in their study (28), subjects were HIV pregnant women.
The finding of no significant effect of immunosuppression and malaria infection on the tested cytokines may be due to the fact that HIV and pregnancy suppress IL-2 production. IL-2 enhances immune response and cytokine production; thus, their suppression may lead to the suppression of other cytokines. This may explain the finding of this study. Parasite density was higher in HIV pregnant women with CD4 T-lymphocytes less than 200 cells/µL as compared with their counterparts presented with a CD4 count above 200 cells/µL in our study. This has been reported (34) among people with HIV (male and female; the females were non-pregnant). However, while Kirinyet (34) showed a significant difference in parasite density in these 2 populations, the difference in this study fails to reach statistical significance.
5.1. Conclusions
Among HIV-infected pregnant women, IL-2 (P = 0.0070), IL-10 (P = 0.0179), and IFN-γ (P = 0.1564) values were lower in primiparous women compared with multiparous women. Married pregnant women with HIV had significantly higher IL-2 (P = 0.0085) and IFN-γ (P = 0.0332) levels compared with those that were single, while marital status did not affect the IL-10 level of pregnant women with HIV. Only IL-2 levels of the HIV-infected pregnant women increased significantly (P = 0.0012) with increasing trimester. The tested cytokines levels were lower in those with malaria compared with those without malaria among HIV-infected pregnant women. The data in this study suggest that HIV status and not malaria infection affects cytokines levels of pregnant women co-infected with HIV and malaria.