1. Background
A sedentary lifestyle and unhealthy dietary patterns are the most important contributors to the worldwide prevalence of obesity (1, 2). The evidence from human and animal studies has shown that the excessive consumption of sugar-sweetened beverages and high-fat diets contribute to adiposity and have been associated with its downstream cardiometabolic complications and obesity-related metabolic disorders (3-6). However, numerous studies have demonstrated that regular exercise training can improve health and prevent the risk of obesity-related diseases and mortality (7-9).
Weight loss and its maintenance are the greatest challenges in the treatment and management of obesity (10). Among exercise interventions, aerobic exercise training appears to be the most effective in the management of obesity and its complications; however, progressive resistance training (PRT) can play a distinct role in this regard. The PRT can increase muscle mass, thereby elevating the resting metabolic rate. Furthermore, PRT may improve total daily energy expenditure by increasing muscular strength that may contribute to more physical activity (11). Therefore, it seems that PRT can play an effective role in overcoming these challenges.
Over the past decade, increasing evidence indicated that skeletal muscle could regulate physiological and metabolic pathways by releasing various bioactive substances, known as myokines (12). Muscle contractions caused by exercise training may affect the production and secretion of myokines in the bloodstream (13). Therefore, PRT may influence the treatment and management of obesity by altering the pool of available myokines.
Myonectin, also known as Fam132b/erythroferrone/C1q/TNF-related protein 15 (CTRP15), is one of the newly discovered myokines that plays an important role in regulating lipid and glucose metabolism (14). Decreased myonectin levels have been shown in obese subjects and type 2 diabetes patients (15). Myonectin expression in skeletal muscle and its circulating levels are increased by endurance exercise (16-19). There have been limited data on the effect of PRT on myokines, especially myonectin.
2. Objectives
Considering the effectiveness of muscle contraction in the synthesis and secretion of myokines, the current study aimed at investigating the effect of PRT on serum myonectin levels on rats fed with a high-fat diet plus sucrose solution.
3. Methods
3.1. Animals
A total of 32 male Wistar rats (187 ± 15 g, 6 - 8 weeks) were obtained from Pasteur’s Institute, Tehran, Iran. The Animals were housed in cages (four rats in each cage) and maintained under controlled light/dark (12/12 h) and temperature (22 ± 2ºC) conditions. After 1 week of acclimation to their living conditions, the animals were initially divided into two groups. One group (n = 16) followed a standard diet (SD) (provided by Behparvar Co., Iran), and the other group (n = 16) followed a high-fat diet (a standard diet with the addition of 12% blend oil) plus a 30% (w/v) sucrose solution (HFDS) in water presented in a second bottle (20, 21). The blend oil for cooking and salad was obtained from Margarine Co., Iran. After 12 weeks of this dietary intervention, each group was equally divided into two groups (sedentary and resistance training).
3.2. Progressive Resistance Training Program
The PRT started 12 weeks after the beginning of the diet program and lasted 8 weeks (one training session per day, 3 days/week). For the accomplishment of this PRT program, a 1-meter ladder was used, which is inclined at 80°. The rats climbed the ladder with weights attached to the base of the tail with tape and clip. The PRT program in the first and second days was accomplished with 50% of the rats’ body weight. The third session consisted of eight ladder climbs (2 min of rest between the repetitions) while carrying progressively heavier loads. The first climb in this session was accomplished with 50% of the animal’s body weight. In the subsequent trials, 10% of the bodyweight was added to the prior weight at the end of each trial. The highest load was considered the rat’s maximum carrying capacity (MCC). Subsequent training sessions consisted of eight ladder climbs. During the first four ladder climbs, the rats carried 50, 75, 90, and 100% of their previous MCC, respectively (22). The subsequent ladder climbs were accomplished by adding 10% of body weight to the MCC of the previous day. Therefore, the daily workload was increased by 10%, compared to that of the previous day.
3.3. Sample Collection
After fasting overnight, 72 h after the last training session, the animals were anesthetized with an intraperitoneal injection of ketamine (50 mg/kg) and xylazine (3 - 5 mg/kg). Approximately 6 mL of the blood was obtained from the abdominal vena cava and centrifuged (3000 rpm; 4 °C; 15 min). The serum was immediately separated and kept frozen at −20°C for further analysis. The flexor hallucis longus (FHL) (i.e., the major muscle recruited in climbing activity) and gastrocnemius (as synergist) muscles were rapidly dissected from the right and left hindlimbs and immediately weighed. Moreover, epididymal and retroperitoneal fats (as visceral white adipose tissue depots) were collected and weighed (23).
3.4. Biochemical Measurements
Serum glucose was determined by an enzymatic-colorimetric method (Pars Azmun Co., Tehran, Iran). Enzyme-linked immunosorbent assay kits were used to assay insulin and myonectin (Hangzhou Eastbiopharm Co., China) concentrations. Homeostasis model assessment of insulin resistance (HOMA-IR) scores were calculated using the following formula: HOMA-IR = [Insulin (mIU/L) × blood glucose (mmol/L)]/22.5 (2).
3.5. Statistical Analysis
All the values were checked for normality using the Kolmogorov-Smirnov test. A two-way analysis of variance was used to determine the main effects of the diet (SD vs. HFDS), training status (sedentary vs. trained), and their interactions. The Pearson correlation method was utilized to examine the simple relationships between serum myonectin levels with other metabolic or body weight parameters. The statistically significant differences were considered in case of a P-value less than 0.05. All the data were statistically analyzed using SPSS software (version 16.0).
4. Results
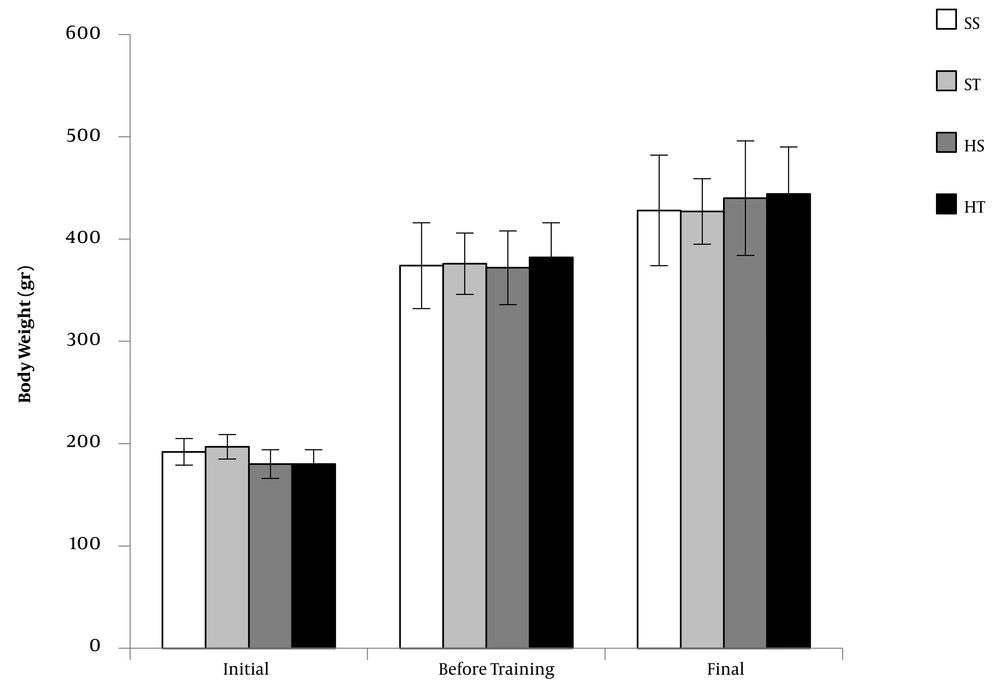
Initial and final body weights were not significantly different between the groups (Figure 1). Weight gain (i.e., final body weight − initial body weight) was higher in HFDS groups than that in the SD groups (P = 0.033; Table 1). The weights of muscles (i.e., FHL and gastrocnemius) and fat pads (i.e., epididymal and retroperitoneal) were expressed as the percentage of body weight. The HFDS rats had lower FHL (P = 0.004) and gastrocnemius (P = 0.009) muscle weights and higher epididymal and retroperitoneal fat weights (P < 0.001) than SD rats (Table 1). The FHL (P < 0.001) and gastrocnemius (P = 0.013) muscle weights were higher, and retroperitoneal fat weights were lower (P = 0.010) in trained groups than those in sedentary groups.
Body weights of rats in three stages, including initial weight (before diet intervention), weight before progressive resistance training program, and final weight. The values are expressed as mean ± standard deviation (n = 8 per group) (SS, standard diet sedentary; ST, standard diet training; HS, high-fat diet plus sucrose sedentary; HT, high-fat diet plus sucrose training).
| Variables | Standard Diet | High-Fat Diet Plus Sucrose | Two-Way ANOVA P-Values | ||||
|---|---|---|---|---|---|---|---|
| Sedentary | Trained | Sedentary | Trained | Diet | Training | Interaction | |
| Weight gain (g) b | 235.6 ± 46.2 | 220.6 ± 22.9 | 262.9 ± 61.9 | 264.1 ± 38.0 | 0.033 | 0.666 | 0.610 |
| FHL/body weight (%) | 0.29 ± 0.02 | 0.33 ± 0.03 | 0.25 ± 0.02 | 0.30 ± 0.04 | 0.004 | < 0.001 | 0.571 |
| Gastrocnemius/body weight (%) | 1.22 ± 0.08 | 1.27 ± 0.10 | 1.10 ± 0.08 | 1.21 ± 0.08 | 0.009 | 0.013 | 0.433 |
| Epididymal fat/body weight (%) | 1.43 ± 0.15 | 1.21 ± 0.14 | 1.97 ± 0.28 | 1.85 ± 0.53 | < 0.001 | 0.159 | 0.657 |
| Retroperitoneal fat/body weight (%) | 1.23 ± 0.19 | 0.84 ± 0.18 | 2.13 ± 0.61 | 1.66 ± 0.56 | < 0.001 | 0.010 | 0.786 |
Body, Muscle, and Fat Weights in Experimental Groups a
After 5 months, HFDS groups had higher serum glucose levels (P < 0.001) and HOMA-IR (P < 0.001), compared to SD groups (Table 2). The HFDS had no significant effects on insulin concentration. Eight weeks of PRT did not significantly affect the serum glucose and insulin concentrations and HOMA-IR in SD and HFDS groups. Serum myonectin levels were lower in HFDS groups than those in SD groups (P < 0.001; Table 2). Myonectin levels were higher in trained groups than those in sedentary groups (P = 0.037).
| Variables | Standard Diet | High-Fat Diet Plus Sucrose | Two-Way ANOVA P-Values | ||||
|---|---|---|---|---|---|---|---|
| Sedentary | Trained | Sedentary | Trained | Diet | Training | Interaction | |
| Serum glucose (mg/dL) | 105.6 ± 11.5 | 107.6 ± 23.8 | 149.7 ± 11.6 | 145.2 ± 24.7 | < 0.001 | 0.857 | 0.646 |
| Serum insulin (mIU/L) | 5.20 ± 0.32 | 4.72 ± 0.65 | 4.99 ± 0.79 | 5.36 ± 0.37 | 0.316 | 0.801 | 0.053 |
| HOMA-IR | 1.36 ± 0.21 | 1.22 ± 0.38 | 1.84 ± 0.30 | 1.91 ± 0.40 | < 0.001 | 0.791 | 0.397 |
| Myonectin (ng/mL) | 1.30 ± 0.13 | 1.34 ± 0.20 | 0.83 ± 0.21 | 1.08 ± 0.13 | < 0.001 | 0.037 | 0.103 |
Serum Concentrations of Myonectin Levels and Metabolic Parameters at the End of the Experiment a
This study examined the simple correlations between serum myonectin levels and other metabolic or body weight parameters (Table 3). Negative correlations were observed between myonectin with glucose levels (r = -0.627; P < 0.001) and HOMA-IR (r = -0.533; P = 0.003). There was a positive correlation between serum myonectin concentration with FHL (r = 0.551; P = 0.002) and gastrocnemius (r = 0.573; P = 0.001) muscle weights. Additionally, negative correlations were noticed between myonectin levels with epididymal (r = -0.563; P = 0.001) and retroperitoneal (r = 0.642; P < 0.001) fat weights.
| Variables | Myonectin (ng/mL) | |
|---|---|---|
| r | P-Value | |
| Glucose (mg/dL) | -0.627 | < 0.001 |
| Insulin (mIU/L) | -0.073 | 0.706 |
| HOMA-IR | -0.533 | 0.003 |
| FHL/body weight (%) | 0.551 | 0.002 |
| Gastrocnemius /body weight (%) | 0.573 | 0.001 |
| Epididymal fat/body weight (%) | -0.563 | 0.001 |
| Retroperitoneal fat/body weight (%) | -0.642 | < 0.001 |
Simple Pearson Correlations Between Serum Myonectin Concentration and Other Variables at the End of the Experiment
5. Discussion
In this study, it was observed that a high-fat diet with sugar solution intake (for a long time; almost 5 months) caused fasting hyperglycemia and elevated HOMA-IR levels in male Wistar rats. Previous studies often used a sugar solution or high-fat diet individually to induce the metabolic syndrome model. Recently, Lozano et al. have investigated the effects of 2 and 8 months of fructose regular consumption, in combination or not with fatty food, on the onset of metabolic syndrome and type 2 diabetes (25). Lozano et al. noticed that only the combination of a fructose solution (25%) and a high-fat diet (21.4% fat) resulted in long-term metabolic disorders.
In addition, in another study, the combination of a sucrose solution (30%) and a high-fat diet (7.5% sunflower oil added to standard diet) caused hyperglycemia and liver steatosis in rats (21). The consequences of this type of diet were weight gain, an increase in the fat pad, and a relative decrease in muscle mass. The diet used in the present study, in accordance with the previous investigations (21, 25), led to weight gain, increased fat pad, and decreased muscle mass.
The relative decrease in muscle mass due to obesity can affect metabolism and other physiological systems. Skeletal muscle, by releasing soluble factors known as myokines, can regulate physiological functions and metabolic pathways in other tissues (13). Myonectin is one of the newly discovered myokines identified as a nutrient-sensitive myokine by Seldin et al. (16). In addition to the physiological importance of myonectin in regulating lipid metabolism, it has been suggested that this myokine plays an important role in linking stress erythropoiesis to iron mobilization in the liver in response to blood loss or anemia (14). Furthermore, myonectin can reduce cardiomyocyte apoptosis and macrophage inflammatory response and plays a crucial role in preventing acute myocardial ischemic injury (17). Therefore, changes in myonectin circulating levels may affect a wide range of physiological functions.
Dietary intervention in the present study decreased serum myonectin levels, which is consistent with the results of a study by Seldin et al. (16). The current study also demonstrated that 8 weeks of PRT increased this myokine to an average level. Furthermore, negative correlations were observed between myonectin concentration with glucose level, HOMA-IR score, and fat weights and positive correlations with FHL and gastrocnemius muscle weights.
To date, few studies have investigated the effect of exercise training on myonectin levels. In animal studies, voluntary running wheel for 2 weeks (16) and endurance treadmill exercise for 4 weeks (17) increased myonectin levels in skeletal muscle and circulation in wild-type mice. Increased myonectin concentrations in the diaphragm muscle of the lean and obese Zucker rats were observed after 9 weeks of aerobic exercises (18). Furthermore, in a human study, 8 weeks of aerobic exercise training increased serum myonectin levels in obese women (19).
Previous studies often focused on low to moderate aerobic exercises. In the present study, high-intensity progressive resistance training (HIPRT), mainly composed of concentric contractions, was used to minimize the muscle damage caused by eccentric exercises. Although the detailed molecular mechanisms of the changes in circulating levels of myonectin by exercise training have not been elucidated, the intensity of muscular contraction can be an effective factor in the production and secretion of this myokine from skeletal muscle tissue.
Seldin et al. indicated that myonectin expression was up-regulated by an increase in cellular cyclic adenosine monophosphate (cAMP) or calcium levels (16). Increased exercise intensity is associated with increased intramuscular calcium and cAMP levels. Therefore, HIPRT may be more effective than moderate- or low-intensity aerobic training in increasing myonectin levels.
In another study, it was shown that myonectin suppressed autophagy in the liver by activating the mammalian target of rapamycin (mTOR) (26). Additionally, other studies revealed that the mTOR complex plays a role in muscle protein synthesis and skeletal muscle hypertrophy (27, 28). Therefore, mayonectin might play an important role in inhibiting muscle protein degradation induced by obesity. In this regard, a positive correlation between myonectin levels and muscle mass was observed in the present study.
5.1. Conclusions
The result of this study demonstrated that serum myonectin levels were decreased in obesity induced by dietary intervention and increased by PRT to average levels. Consequently, the results of the current study suggest that PRT may be an efficient intervention to enhance serum myonectin levels, which is associated with an increase in muscle mass and the improvement of body composition.