Introduction
Respiratory distress syndrome (RDS) is one of the most common causes of morbidity in preterm neonates [1]. The mainstay of management is supportive care with mechanical ventilation and high concentrations of inspired oxygen.
Because mechanical ventilation is associated with morbidity, the trend is to minimize the use of mechanical ventilation [2]. The best treatment of RDS in preterm neonates needs to be further investigated [3].
Mechanical ventilation induced lung injury is characterized by excessive tidal (volutrauma) [4], shear injury related repetitive cycling of distal airways at suboptimal lung volumes [5], and the consequent release of biochemical substances that instigate pulmonary inflammation [6].
Endotrauma is the name given to injury to the airways and lungs from the disruption of homoeostasis that occurs during, and sometimes after, artificial ventilation through and endotracheal tube (ETT) [7]. The ETT is probably a major factor adding to causal respiratory failure and injury during invasive ventilation. Endotracheal intubation is a traumatic and painful procedure that requires sedation and can be associated with hemodynamic instabilities, airway emergencies acute air flow, and increased work of breathing [8].
The ETT bypasses the glottis and hinders the neonate’s adaptive mechanism (grunting) [9]. For preserving the end-expiratory lung volume the ETT also provides a direct rout in to the lower, sterile airway, which increases the risk of ventilator-associated pneumonia and sepsis [10].
Nasal continuous positive airway pressure (NCPAP) or nasal intermittent positive pressure ventilation (NIPPV) is an alternative to invasive ventilation that does not require an ETT and permits spontaneous breathing during continuous pressure applied with prongs in the nares. NCPAP and NIPPV are 2 noninvasive treatments for RDS. NCPAP was shown to be effective in treating infants with RDS. It enables the avoidance of mechanical ventilation in a relatively large number of infants [11-13]. NCPAP is a form of continuous distending pressure (CDP) which is defined as the maintenance of increased transpulmonary pressure during the expiratory phase of respiration. The basic goal is to provide distension of the lungs, thereby preventing collapse of the alveoli and terminal airways during expiration.
By using NCPAP, up to 40% neonates with RDS may need intubation and ventilation [14]. In NIPPV the positive pressure ventilator breath is delivered only after initiation of respiratory effort by the infants, when the glottis is likely to be open or after an apnea interval [15]. There is little study in literature comparing the early use of NIPPV with NCPAP as primary modes of respiratory support. We aimed to determine whether NIPPV and NCPAP would have different survival rates and possible complications.
Materials and Methods
This study was a prospective clinical trial conducted at a level III neonatal care unit of Afzalipour hospital between January and May 2012 in Kerman University of Medical Sciences; Iran, an excellent center for high risk pregnancies. The aim of this study was to compare the effectiveness of NIPPV and NCPAP in the treatment of neonates with RDS.
Based on setting the power and type one error at 80% and 5%, we have estimated that the total number of patients required was 120 (i.e. 60 per treatment group).
All of the patients were inborn (gestation 28 to 36 weeks) with birth weight between 1000 and 3000 g who had respiratory distress.
Infants who had significant morbidity apart from RDS [including cardiac disease (not patent ductus arteriosus)], congenital malformation (including congenital diaphragmatic hernia, tracheoesophageal fistula and cleft lip/palate), and the infants who had cardiovascular or respiratory instability because of sepsis, anemia, or severe intraventricular hemorrhage (IVH) on admission were excluded from the study.
Early nasal respiratory support (NCPAP or NIPPV) was initiated in spontaneous breathing premature infants who showed signs of respiratory distress (presence of ≥2 features of retraction, grunting, and respiratory rate >60/min) within 6 h of birth and a Silverman-Anderson retraction score of 6 or 7. In case of nasal respiratory support indication, the mode was randomized between NCPAP and NIPPV
To randomly assign patients in 2 treatment groups, the minimization technique was applied with respect to baby's gender and birth weight (≤1500 vs. >1500 g). By implementing this method, we balanced the gender and weight distribution in treatment groups. In both groups of NCPAP and NIPPV nasopharyngeal tube was implemented. Survanta (Abbott laboratories S.A.) 100 mg/kg/dose, 1 to 2 doses as needed, was used in both groups with INSURE technique.
Both modes of nasal respiratory support were delivered by the event medical ventilator (Inspiration LS infant, Ireland) via nasopharyngeal prongs. Subjects in the NCPAP group were initiated on 5 cm of water and flow 6-7 L/min. The maximum permissible setting were CPAP 7 cmH2o and fraction of inspired oxygen (FIO2) 0.6.
Subjects in NIPPV (NCPAP+) group were initiated on peak inspiratory pressure (PIP) 11 cm of water, peak end expiratory pressure (PEEP) 5 cm of water, I:E (Inspiration:Expiration) 1:5.7, flow 6-7 L/min, and rate 15/min (default of event ventilator on NCPAP+ mode). The maximum permissible PEEP was 7 cm of water and FIO2 was 0.6. Targeted saturation was 88-92%. Settings in both groups were adjusted based on arterial blood gases (ABG) and pulse oximetry.
During NIPPV and NCPAP infants were cared in high dependency area of the NICU with 24 h monitoring of vital signs, saturations, and signs of clinical improvement of deterioration in the respiratory status. Big bore orogastric tube was inserted in all infants. NCPAP or NIPPV was considered to be successful if the respiratory distress improved and the baby could be successfully weaned off NCPAP or NIPPV.
The criteria for weaning was absence of respiratory distress (minimal or no retractions and respiratory rate between 30 and 60/min) and SpO2> 90% on FIO2<0.3 and PEEP <5 cm of water.
Mechanical ventilation were considered for failure of NCPAP or NIPPV in babies with Pao2 <50 mmHg or PaCO2>60 mmHg and PH <7.25 with FIO2>0.6 or those with clinical deterioration (increased respiratory distress) including severe retraction on PEEP >7 cm of water or recurrent apnea (>2 episodes within 24 h associated with bradycardia) requiring bag and mask ventilation.
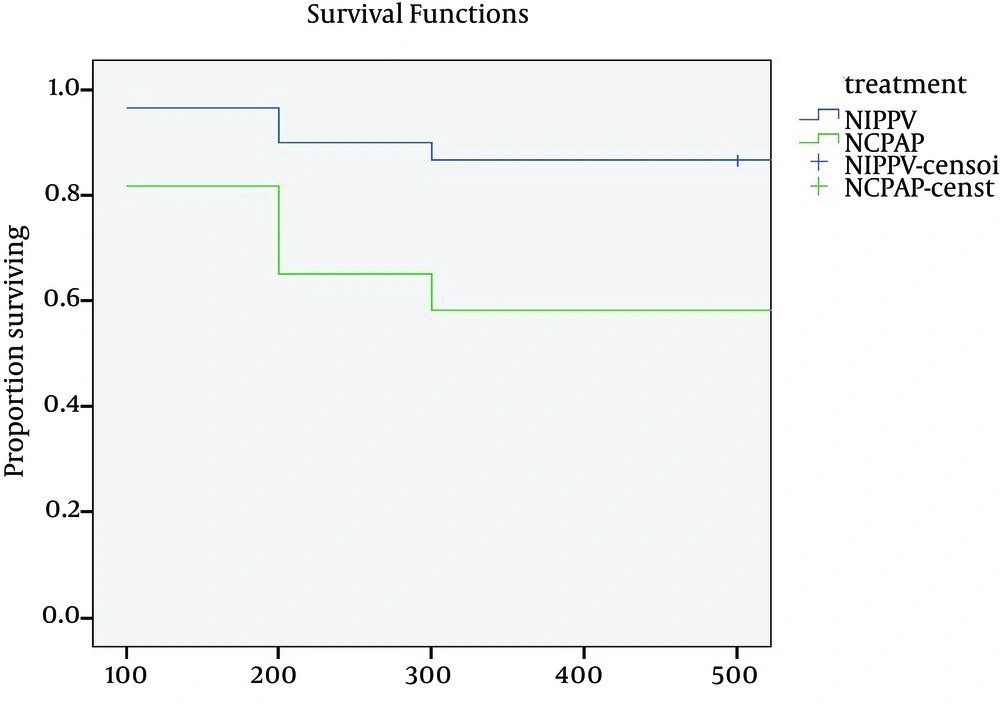
The primary outcome measure was the percent of infants in whom nasal respiratory support failed and who needed endotracheal ventilation. Infant variables evaluated included weight, gestational age, Apgar score at minute 1 and minute 5, and FIO2 requirement. The main outcome of this study was the failure rate of response to treatment. The survival rates are compared in 2 arms at different hours (Table 1).
We also compared treatment options in terms of duration of oxygen, duration of hospital stay, time to start feeding, time until full feeds, mean initial PEEP, mean initial FIO2, and time to stay at hospital and costs. We reported the incidence of neonatal morbidities in 2 treatment groups: pneumothorax, PDA by echocardiography (ACCUVIX 10, Medison, Korea), IVH by cranial ultrasonography (ACCUVIX 10, Medison, Korea), severe IVH (grade 3, 4), and death 7 days from weaning.
We applied actuarial life table method to estimate survival rates. Survival rates were reported for every 12 h up to 72 h (3 days).
To display the result graphically, Kaplan-Meier curves are plotted. For all tests the level of significance was set at p<0.05. Cox regression model was fitted to estimate risk of failure for treatment while adjusting the effect of other variables.
The study protocol was approved by local ethical committee of Kerman University of Medical Sciences (Ethic code: K-90-328). All the parents signed informed consent before participating in the study. This study has been registered in Iranian Registry Clinical Trial (IRCT 201202273250N6).
Data were analyzed applying t-test and χ2 tests for comparison of continuous and categorical variables between 2 groups.
Results
A significant difference was seen between groups in terms of PEEP and FIO2. The mean±SD duration of nasal support was 47.20±20.71 h for NIPPV and 61.20±29.45 h for NCPAP, which was found to be statistically significant, giving a p=0.003 (Table 1).
We found a significant difference for the mean±SD to start feeding between NIPPV and NCPAP 1.88±0.94 and 2.48±0.96 days, (p=0.001) (Table 1).
The NIPPV and the NCPAP groups had comparable demographic characteristics (Table 2). For example, the difference between mean gestational ages was 0.11 week (32.16 vs. 32.05).
The mean±SD of initial PEEP in NIPPV and NCPAP groups were 5.31±0.56 and 5.9±0.81 cmH2o, (p <0.001) (Table 2).
The mean±SD of initial FIO2 in NIPPV and NCPAP groups were 52±2.94% and 54.41±3.69%, (p <0.001) (Table 2).
Also, we found a significant difference for the mean±SD time to full feed between NIPPV and NCPAP 5.96±1.74 and 8.23±2.38 days, (p=0.001) (Table 2).
The mean±SD duration of hospital stay in NIPPV and NCPAP groups were 7.45±2.02 and 9.65±2.49 days, (p=0.001) (Table 2).
The mean±SD cost of hospitalization in NIPPV and NCPAP groups were 865.9±313 $ and 1032.2±354 $, (p=0.007) (Table 2).
A significant difference was seen between groups in terms of the mean duration of hospital stay and the mean cost of hospitalization. The percent of receiving antenatal steroid in NIPPV and NCPAP groups were (83.3%) and (73.3%) (Table 2).
The percent of receiving surfactant in NIPPV and NCPAP groups were (48.3%) and (40%) (Table 2).
However, no difference was seen between groups in terms of receiving antenatal steroid and receiving surfactant.
Failure of nasal support (need for endoteracheal ventilation) was higher after initial treatment with NCPAP (41.7%) compared with NIPPV (13.3%), giving a p<0.001 (Table 2).
| Group | 12 h | 24 h | 36 h | 48 h | 60 h | 72 h |
|---|---|---|---|---|---|---|
| NIPPV (%) | 100 | 97 | 97 | 90 | 90 | 87 |
| NCPAP (%) | 100 | 82 | 82 | 65 | 65 | 58 |
Comparison of estimated success rate in NIPPV vs. NCPAP
| Variable | NIPPV (N=60) | NCPAP (N=60) | p-Value | |
|---|---|---|---|---|
| Gender | Male [N(%)] | 32 (53.3) | 31 (51.7) | 0.85 |
| Female [N(%)] | 29 (49.7) | 29 (48.3) | ||
| Gestational age (week) (Means±SD) | 32.16±2.08 | 32.05±2.94 | 0.80 | |
| Birth weight (g) (Means±SD) | 1882.50±56 | 1807.05±52 | 0.45 | |
| Born by cesarean section [N(%)] | 49 (81.7) | 42 (70) | 0.13 | |
| Apgar score at 1 minute (Means±SD) | 8.18±0.83 | 8.38±0.71 | 0.16 | |
| Apgar score at 5 minute (Means±SD) | 9.50±0.59 | 9.6±0.58 | 0.28 | |
| Antenatal steroid received [N(%)] | 50 (83.3) | 44 (73.3) | 0.18 | |
| Surfactant received [N(%)] | 29 (48.3) | 24 (40) | 0.35 | |
| Initial PEEP (cmH2o) or CPAP (Mean±SD) | 5.31±0.56 | 5.9±0.81 | <0.001 | |
| Initial FIO2 (%) (Mean±SD) | 52±2.94 | 54.41±3.69 | <0.001 | |
| Response to treatment (%) | No (failure) [N(%)] | 8 (13.3) | 25 (41.7) | 0.001 |
| Yes (success) [N(%)] | 52 (86.7) | 35 (58.3) | ||
| Time of stop nasal support (h) | 47.20±20.71 | 61.20±29.45 | 0.003 | |
| Time to start feeding (day) | 1.88±0.94 | 2.48±0.96 | 0.001 | |
| Time to full feed (day) | 5.96±1.74 | 8.23±2.38 | <0.001 | |
| Time oxygen received (day) | 4.19±5.05 | 4.85±1.32 | 0.35 | |
| Time stay hospital (day) | 7.45±2.02 | 9.65±2.49 | <0.001 | |
| Cost ($) * 1$=10000 R | 865.9±313 | 1032±354 | 0.007 | |
| Complication | PDA (N) | 3 | 4 | 0.69 |
| IVH (N) | 2 | 3 | ||
Patient characteristics in NIPPV and NCPAP modes
We compared success rates in 2 treatment arms at every 12 h (Table 1). In the first 24 h, the difference between success rates was about 15% (97% in NIPPV versus 82% in NCPAP), but at the end of the 3rd day it was about 29 % (87% in NIPPV versus 58% in NCPAP) (Table 1, Fig. 1). The log-rank test confirmed a significant difference between the survival curves. This graph indicates that the first hours of treatments are critical.
Finally, we developed a Cox regression model to adjust the effect of treatment regarding sex, delivery, gestational age, steroid, and surfactant. We have seen that the risk of failure in NCPAP was 4.23 time higher relative to NIPPV (Table 3). We also saw that administering steroid in NIPPV groups was associated with about 80% reduction in risk of failure (p=0.04), (Table 3).
| HR | CI | p-Value | ||
|---|---|---|---|---|
| lower | upper | |||
| Treatment | 4.229 | 1.86 | 9.58 | 0.001 |
| Sex | 1.466 | 0.710 | 3.02 | 0.301 |
| Delivery (N) | 1.78 | 0.622 | 5.131 | 0.281 |
| Gestational (N) | 0.926 | 0.819 | 1.046 | 0.216 |
| Steroid (mg/day) | 0.186 | 0.037 | 0.938 | 0.042 |
| Surfactant (mg/kg) | 0.588 | 0.259 | 1.338 | 0.206 |
Cox regression
The reasons of failure in the NIPPV group were recurrent apnea in 4 cases, increased FIO2 in 3 and frequent desaturation in one patient. The reasons for failure in the NCPAP group were: 11 had recurrent apnea, 7 had frequent desaturation and 7 had increased FIO2.
A total of 7 neonates had PDA (3 in NIPPV group and 4 in NCPAP group), and 5 neonates had IVH (2 in NIPPV group and 3 in NCPAP group) that resolved in repeated head ultrasound scanning (Table 2). None of the babies developed pneumothorax.
At next step, we focused on patients who received NIPPV and NCPAP separately to compare characteristics of patients who respond/not respond to the treatment. As shown in table 4, there was no significant difference between sex, gestational age, and birth weight of neonates who failed NIPPV treatment comparing the success group.
| Variable | NIPPV | |||
|---|---|---|---|---|
| Success (N=52) | Failure (N=8) | p-Value | ||
| Gender [N(%)] | Male | 28(53.8) | 4 (50) | 0.83 |
| Female | 24 (46.2) | 4 (50) | ||
| Gestational age (week) [N(%)] | 28-31 | 19 (36.5) | 5 (62.5) | 0.16 |
| 32-36 | 33 (63.5) | 3 (37.5) | ||
| Birth weight (g) [N(%)] | 1000-1500 | 16 (30.8) | 5 (62.5) | 0.08 |
| 1501-3000 | 36 (69.8) | 3 (37.5) | ||
Patient characteristics by failure of treatment in NIPPV group
Also, we found that in NCPAP, the failure rate was significantly high in the subgroups of gestational age <32 W and birth weight <1500 g (Table 5).
| Variable | NCPAP | p-Value | ||
|---|---|---|---|---|
| Success (N=35) | Failure (N=25) | |||
| Gender [N (%)] | Male | 18 (51.4) | 13 (52) | 0.69 |
| Female | 17 (48.6) | 12 (48) | ||
| Gestational age (week) [N(%)] | 28-31 | 8 (22.9) | 20 (80) | <0.001 |
| 32-36 | 27 (77.1) | 5 (20) | ||
| Birth weight (g) [N(%)] | 1000-1500 | 8 (22.9) | 18 (72) | <0.001 |
| 1501-3000 | 27 (77.1) | 7 (28) | ||
Patient characteristics by failure of treatment in NCPAP group
Discussion
In this study, the use of early NIPPV decreased the need for intubation as compared to early NCPAP in preterm neonates of 28-36 weeks gestation with respiratory distress. NIPPV was well tolerated. The primary outcome was evaluated by 72 h because the need for intubation beyond 72 h is often unrelated to RDS. A few studies have found that NIPPV was superior to NCPAP post extubation, after mechanical ventilation and surfactant treatment, for RDS and for apnea of prematurity.
We demonstrated that NIPPV was more successful than NCPAP as the initial treatment of respiratory distress in premature infants by reducing the rate of endotracheal ventilation, and lessening the mean of initial PEEP, initial FIO2, time to start feeding, time to full feed, time to stop nasal support, hospital stay and the mean cost of hospitalization. In the other RCT, in which NIPPV and NCPAP as a primary mode were compared, the mean gestational age was similar to our study [2]. The number of failures was less in the NIPPV group compared to the NCPAP group in our study (13.3 vs. 41.79). Failure rate of NIPPV group vs. NCPAP in the other RCT was (25% and 49% respectively) [2].
Our study also correlated failure of nasal support with birth-weight and gestational age. In Kugleman's study failure of nasal respiratory support was significantly associated with lower birth weight [2].
The use of the experience of medical teams with nasal support is increasing in recent years. Our study represents our single center experience. In Kugleman et al. study, the length of hospital stay, time to start feeding, time to full feed and time to stop nasal support in the NIPPV and NCPAP groups were comparable [2]. In Sai Sunil Kishore et al. study, the length of hospital stay, time to full feed and time to stop nasal support in the NIPPV and NCPAP groups were not significantly different [14]. The existing difference may have arisen because of the shorter duration of nasal support in NIPPV and the use of mini mal enteral feeding technique in our study. Moreover, our sample size was larger than the previous study.
Our results showed that the effect of NIPPV was not modified by gestational age, birth weight, gender, and surfactant usage as well. The baseline risk of failure in NCPAP was lower in more mature babies. NIPPV on the other hand, provides an inspiratory positive pressure for ventilatory assistance, lung recruitment, and an expiratory positive pressure to help recruit lung volume, and adequate lung expansion. Moretti et al. found that application of NIPPV was associated with increased tidal volume and minute volume as compared to NCPAP [16].
The other expected advantage for NIPPV over NCPAP is the elimination of PCO2 by providing rates [16-18].
Noninvasive ventilation (NIPPV) is a form of respiratory assistance that provides a greater level of respiratory support than dose NCPAP and may prevent intubation in a larger fraction of neonates who would otherwise fail CPAP. NIPPV may have advantages over NCPAP in stabilizing a borderline functional residual capacity, reducing dead space, preventing atelectasis, and improving lung mechanics [19, 20]. We conclude that among preterm neonates presented with respiratory distress within 6 h of life, NIPPV is more efficacious than early NCPAP in reducing the need for intubation and invasive ventilation. Early NIPPV is safe and does not increase complications. In particular, we recommend the use of early NIPPV in neonates of 28-30 weeks gestation and even those who do not receive surfactant. It is proposed that NIPPV be evaluated in neonates born at less than 28 weeks of gestation. Early intervention with NIPPV may result in preventing undue intubation and its associated risk.