Introduction
Acinetobacter baumannii is an opportunistic pathogen involved in hospital outbreaks worldwide [1]. In the last two decades, A. baumannii has become more prevalent as an opportunistic pathogen and an important species implicated in nosocomial infection, causing pneumonia, septicemia, urinary tract infections, and wound infections [2-3]. Reports of multidrug-resistant isolates have increased during the last years, which have in turn led to an increased use of broad-spectrum antibiotics [1]. Treatment of infections due to this microorganism poses a major clinical challenge [4].
The most of the expanded-spectrum β-lactamases of Acinetobacter and other Gram negative bacteria are the clavulanic acid-inhibited extended-spectrum β-lactamases (ESBLs) of Ambler class A that have been reported extensively and are widespread [1, 5]. Extended-spectrum β-lactamases are capable of hydrolyzing extended-spectrum cephalosporins with an oxyimino side chain and its activity is well inhibited by clavulanic acid, sulbactam and tazobactam. These cephalosporins include cefotaxime, ceftriaxone, ceftazidime and cephpodoxime [6].
The aim of the present study was to investigate the prevalence of ESBL and clonal relatedness of A. baumannii isolates identified in the intensive care unit, pediatric, emergency and infectious disease ward of the Taleghani, Imam Reza and Imam Khomeini hospitals in Kermanshah, Iran.
Materials and Methods
In the present study, A. baumannii isolates collected from patients admitted to Kermanshah hospitals between March 2010 and December 2011 were included. These strains were recovered from sputum, blood and urine. The specimens were sub cultured by swabbing in the microbiology laboratory and transported in transport culture media to Research Laboratory, School of Medicine, Kermanshah, for further analysis. Bacteria were identified by biochemical tests such as oxidase, TSI, SIM and OF tests and A. baumannii strains were confirmed by the API20NE kit (version 6.0, bio-Merieux, Marcy L'Etoile, France).
Antimicrobial susceptibility testing was performed using the disk diffusion method according to the CLSI guidelines [7]. The agents tested included amikacin (AN: 30 μg), ceftriaxone (CRO: 30 μg), ciprofloxacin (CIP: 5 μg), trimethoprim/sulfamethoxazole (TS: 30 μg), gatifloxacin (GAT: 5 μg), colistin (CL: 10 μg), gentamicin (GM: 10 μg), imipenem (IMP: 10 μg), meropenem (MEM: 10 μg), piperacillin (PRL: 100 μg), piperacillin-tazobactam (PT:100/10 μg), polymyxin B (PB: 300 unit), levofloxacin (LVF: 5 μg), minocycline (MIN: 30 μg), mezlocillin (MEZ: 75 μg), tetracycline (TET: 30 μg), tobramycin (TOBI: 10 μg), cefepime (CEF: 30 μg), cefpodoxime (CPD: 10 μg), cefotaxime (CTX: 30 μg), ceftazidime (CAZ: 30 μg), rifampicin (RF: 5 μg) (MAST, Merseyside, U.K).
ESBL screening: Cephalosporin-resistant (resistant to at least one of them including cefotaxime, cefepime, ceftazidime and cefpodoxime) isolates were screened for ESBL production. The double disk method was used for detection of ESBL isolates. In this method, a disk of ceftazidime (30 μg), cefotaxime, ceftriaxone or cefepime alone (30 μg) or in combination with clavulanic acid (30μg/10 μg) were tested on a Mueller-Hinton plate. Increase of more than 5 mm in diameter in the presence of clavulanic acid was interpreted as positive [8].
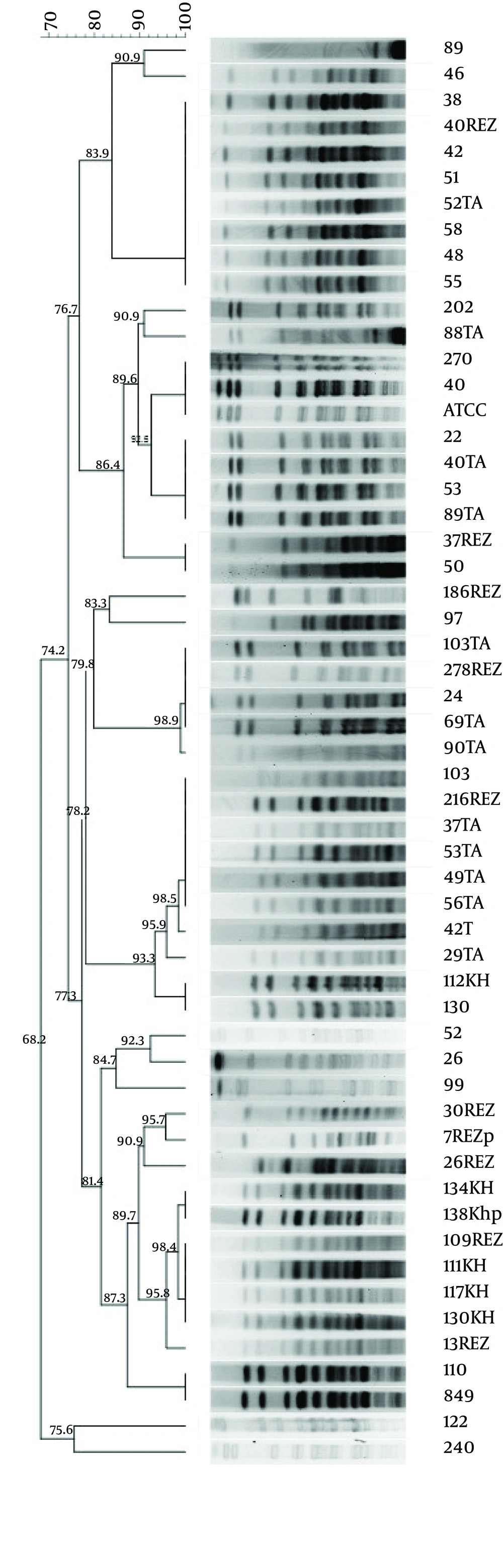
Pulsed-field gel electrophoresis: Pulsed-field gel electrophoresis (PFGE) was performed on all ESBL-positive A. baumannii isolates (N=52) using restriction enzyme ApaI (New England Biolabs, Ipswich, MA, USA) as described by Durmaz et al. [9] using A. baumannii ATCC 19606 as the internal reference strain and Lambda Ladder PFG Marker (NEB). Electrophoresis was performed in a pulsed-field electrophoresis system (Chef Mapper; Bio-Rad Laboratories, Hercules, CA, USA) with the following conditions: temperature 14°C; voltage 6 V/cm2; switch angle 120°; switch ramp 2.2-35 s for 20 h. The images obtained were processed by Bionumerics 7.0 software (Applied Maths NV, St-Martens-Latem, Belgium). Pulsotypes were considered to represent the same clones or classified as the same type when the pattern similarity was > 80% (Fig. 1) [10-11].
Statistical analysis: Data were entered into a database and then statistical analyses were performed using SPSS-16.0. Mann-Whitney U test was used for comparing differences of ESBL positivity between groups. A p-value <0.05 was considered to indicate significance [12].
Results
A total of 84 isolates of A. baumannii were obtained from Kermanshah hospitals during the study period, of which 52 were confirmed as ESBL producers. Among the 52 ESBL-producing isolates, 19 isolates were obtained from Imam Reza hospital, 27 isolates from Taleghani and 6 isolates from Imam Khomeini hospital and respectively isolated from intensive care unit (ICU) (59.6%), urgency (17.3%), infant (neonatal) (13.5%) and pediatric wards (9.6%). The sources included blood (28.8%, N=15), sputum (57.6%, N=30) and urine (13.4%, N=7). The mean age of the men with A. baumannii isolates were 32.76±23.31 years and the women were 28.23±27.4 years. This study showed high rates of resistance to ampicillin and cephpodoxime (98.1 and 92.3%) and also to other antibiotics (Table 1). According to these results, most isolates were susceptible to colistin and tigecycline with low resistance rates of 7.7% and 3.8%, respectively, but had significant resistance rates to polymyxin B (11.5%) and minocycline (17.3%) (Table1)
The 52 ESBL isolates were clustered into eight pulsotypes: A (N=9), B (N=10), C (N=2), D (N=5), E (N=9), F (N=15), G (n=1) and H (N=1). The pulsotype F was dominant in different wards of our hospitals, sharing approximately 85% similarity within the cluster. Most of the resistant isolates belonged to to clones A, B and F.
| Antimicrobial | Susceptibility; no. (%) of isolates: | ||
|---|---|---|---|
| Susceptible | Intermediate | Resistant | |
| 3 (5.8) | 4 (7.7) | 45 (86.6) | |
| 2 (3.8) | 3 (5.8) | 47 (90.4) | |
| 23 (44.2) | 0 | 29 (55.8) | |
| 24 (46.2) | 0 | 28 (53.8) | |
| 20 (38) | 3 (5.8) | 29 (55.7) | |
| 48 (92.3) | 0 | 4 (7.7) | |
| 6 (11.5) | 1 (1.9) | 45 (86.5) | |
| 8 (15.4) | 1 (1.9) | 43 (82.7) | |
| 10 (19.2) | 3 (5.8) | 39 (75) | |
| 10 (19.2) | 2 (3.8) | 40 (76.9) | |
| 46 (88.5) | 0 | 24 (46.2) | |
| 10 (19.2) | 0 | 42 (80.7) | |
| 18 (34.6) | 4 (7.7) | 30 (57.7) | |
| 41 (78.8) | 2 (3.8) | 9 (17.3) | |
| 8 (15.4) | 4 (7.7) | 40 (76.9) | |
| 17 (32.7) | 1 (1.9) | 34 (65.4) | |
| 17 (32.7) | 2 (3.8) | 33 (63.5) | |
| 50 (96.2) | 0 | 2 (3.8) | |
| 42 (80.8) | 0 | 10 (19.2) | |
| 4 | 0 | 48 (92.3) | |
| 26 (50) | 0 | 26 (50) | |
| 7 (13.5) | 3 (5.8) | 42 (80.8) | |
| 1 (1.9) | 0 | 51 (98.1) | |
| 29 (55.8) | 0 | 23 (44.2) | |
Discussion
Treatment of infections caused by ESBL-producing A. baumannii has emerged as an important challenge. ESBL-producing A. baumannii strains have been widely reported all over the world, such as Palestine, Europe, North America, and China also reported of Iran [13]. In the western regions of Iran, there has been a marked rise in laboratory reports of A. baumannii from 2010 through 2012, where most infections occurred in intensive care unit (ICU). This study showed that resistance is also more pronounced in the intensive care unit. Resistance factors in increasing the number of isolates in this ward were such that: Long-term hospitalization in this ward, use the last line drugs (including third-generation cephalosporins), transfer plasmids containing antibiotic resistance genes to susceptible isolates, stability of this resistant isolates by transmission of patient to patient. In our study, all isolates were A. baumannii, Meric et al. was similar with the study [14].
An outbreak of Acinetobacter respiratory tract infection resulting from ventilator equipment that was reported by Cefai et al. [15], also in our study showed that the highest number of isolates related to sputum 57.6% (N=30). Two previous studies on A. baumannii in Iran showed that 2-21% were ESBL-producing isolates [13, 16]. While the study designs differ, the rate of ESBL-producing isolates was much higher in our study, suggesting that further resistance to these antibiotics may have developed in the meantime. Two different studies in Korea and Turkey showed an incidence of 54.6% and 46% ESBL producers, respectively [17-18], similar to our study. Colistin and tigecycline are considered as a viable therapeutic option in the treatment of infection due to ESBL-producing A. baumannii especially in intensive care units. Some studies showed that ESBL-producing strains could be carrying genes coding for resistance to these antibiotics [19], therefore, genetic research will be needed for the detection of genes. This finding suggests that genes coding for ESBLs and genes coding for resistance to these antibiotics may reside within the same plasmids and therefore spread together.
Pulsed-field gel electrophoresis (PFGE) is the gold standard technique to investigate the moleucular epidemiology of bacteria. The PFGE profiles A, B and F was believed to be endemic in the ICU, emergency, pediatric and infection area throughout the years. The clones A, B and F were resistant to polymyxin B and colisitin that may suggest that they may share a common origin. The clones B and D were resistant to cephalosporins (cefpodoxime, cefepime, cefotaxime and ceftazidime); they were isolated from a similar hospital (Hospital 1). The clone E spread in three hospitals and shared similar resistance patterns to antibiotic agent (Table 2), the results suggest that they originate from a common source.
| Hospital | Isolate N. | Susceptibility profiles | PFGE group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMPS | ECAZ | ECPM | ECTX | ECPD | PB | CO | TGC | |||
| 8 | 1 | 6 | 8 | 2 | 0 | 1 | 1 | 0 | A | |
| 8 | 2 | 5 | 7 | 3 | 2 | 2 | 1 | 1 | B | |
| 3 | 2 | 3 | 2 | 2 | 2 | 0 | 0 | 0 | D | |
| 5 | 2 | 3 | 5 | 1 | 0 | 0 | 0 | 0 | E | |
| 3 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | F | |
| 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | A | |
| 2 | 2 | 1 | 2 | 2 | 0 | 0 | 1 | 0 | B | |
| 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | C | |
| 2 | 2 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | D | |
| 3 | 2 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | E | |
| 7 | 3 | 4 | 4 | 4 | 0 | 1 | 1 | 1 | F | |
| 1 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | G | |
| 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | H | |
| 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | E | |
| 5 | 4 | 2 | 3 | 5 | 0 | 2 | 0 | 0 | F | |
a AMPS: Ampicillin/Sulbactam; ECAZ: ESBL-Ceftazidime; ECPM: ESBL-Cefepime; ECTX: ESBL-Cefotaxime; ECPD: ESBL-Cephpodoxime; PB: Polymyxin B; CO: Colistin; TGC: Tigecycline; ESBL Template: A series of total ESBLs (CAZ, CPM, CTX and CPD); 1= Taleghani hospital ; 2= Imam Reza hospital and 3= Imam Khomeini hospital
In conclusion; early and timely detection of ESBL-producing A. baumannii clones is useful for preventing their spread within the hospital. Tigecylcine and colistin remain as the therapeutic options for the treatment of infections caused by A. baumannii. PFGE analysis is helpful for detection of common strains in different wards and prevention of further spread of these pulsotypes to other hospital environment.