Introduction
The herbicide parquet (1, 1-dimethyl-4, 4-bipyridilium dichloride: PQ) increases agricultural yield by killing weeds worldwide [1]. It is a fast acting, non-selective compound, which destroys tissues of green plants on contact and by translocation within the plant [2]. In humans, intentional or accidental ingestion of PQ is frequently fatal as the result of multiorgan failure [1]. PQ has been demonstrated to be a highly toxic compound for humans and animals and many cases of acute and chronic poisoning and death have been reported over the past few decades [3]. Based on the role of reactive oxygen species (ROS), antioxidants may be an important tool against PQ-induced toxicity due to the lack of effective treatment or a specific antidote [4]. The effects of PQ on antioxidant system and the role of antioxidants in PQ toxicity have been evaluated [5, 6]. The overall impact of environmental changes on the mechanisms of PQ toxicity and effective treatment is poorly understood yet. Despite the lack of a strong evidence base or consistent recommendations, any rational approach to treatment should consider both the mechanism of PQ toxicity and accepted treatment of patients with PQ.
Materials and Methods
Bibliographic databases including Pub Med, Scopus and Google Scholar were searched between 1960 to 2013 for the keywords "parquet" toxicity and "oxidative stress". In the first step, 378 articles were found, after elimination of duplicates or irrelevant papers 70 papers were selected and reviewed. Reference lists of published articles were hand-searched to ensure inclusion of all possible studies drawn in figure 1.
Results
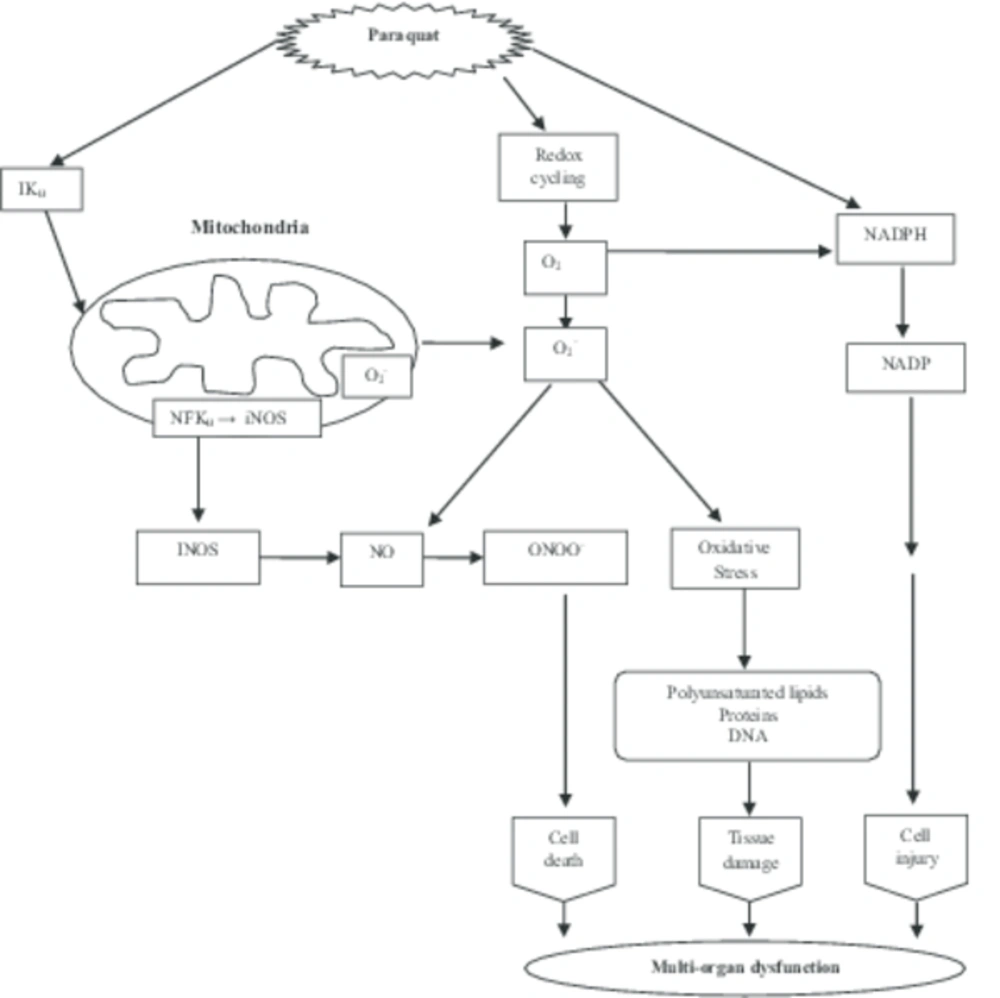
Role of ROS in PQ-mediated multi organ toxicity: PQ an environmental herbicide/pesticide causes toxicity through the generation of ROS [4, 7, 8], and formation of apoptosis related molecules. PQ promotes intracellular generation of ROS via 3 distinct pathways: 1- reduction of PQ by NADPH-cytochrome P450 reeducates and a subsequent redo cycle with involvement of superoxide dismutase (SOD) and glutathione pools, 2- inhibition of mitochondrial electron transport chain, and 3- interaction with other enzymes such as nitric oxide syntheses (cytosolic), NADPH oxidize (plasma membrane), thioredoxin reeducates (cytosolic form, Tax 1), and xanthenes oxidize [9]. PQ-induced oxidative stress has been reported to be linked to endoplasmic reticulum stress signaling pathways and subsequent formation of capsize dependent apoptosis related molecules [10]. The reduced PQ radical is immediately deoxidized by molecular oxygen, resulting in the formation of the superoxide anion radical (O2-). Superoxide then dismutase’s to hydrogen peroxide, thus initiating the process of tissue damage [11] by exerting pro-oxidant effects or inhibiting defense systems [12] (Fig. 2).
This resulted in the production of other ROS that are highly reactive to cellular macromolecules, leading to oxidative stress [13]. The importance of oxidative stress as a mechanism of PQ toxicity has been demonstrated in studies in plants [14], bacteria [15], in vitro [16] and in vivo systems [4, 17]. The involvements of ROS and lipid per oxidation have been implicated in the toxicity of PQ [18] as well as organ chlorine [19] and organophosphate pesticides in human [20] and animals [21].
This preliminary investigation suggests that PQ formulating workers are exposed to more oxidative stress as evidenced by increased concentration of plasma lipid per oxidation (LPO) and changes in antioxidant status [4]. The pulmonary, liver, kidney and brain toxicity caused by PQ [22].
Early mortality of patients with PQ poisoning occurs as a result of vascular collapse, and delayed mortality is mainly due to progressive pulmonary fibrosis [23]. The mechanism of PQ toxicity in various species has been investigated and is attributed to its redo cycling properties [5].
Neurotoxicity during PQ exposure: Many cases of acute PQ poisoning and death have been reported over the past few decades. Animal studies have demonstrated that PQ can cause neurodegeneration of dopaminergic neurons [24]. However, there is still a lot of controversy regarding whether the neurotoxicological actions of PQ represent an accurate experimental model for studying the pathogenesis of disease such as Parkinson disease (PD) [25].
Previous studies have shown that PQ deposition is found in hippocampus as well as in frontal cortex (FC) [26]. Psychotic nuclei appeared in substantia nigra (SN), in FC and in hippocampus of the treatment group, indicating that, apart from SN, hippocampus and FC are also affected by PQ mediated neurotoxicity. PQ exposure induced the formation of α-syncline containing deposits [7].
Nevertheless, the study of PQ’s neurotoxin properties has provided valuable information regarding the potential mechanisms involved in the progression of neurodegeneration associated with environmental toxicity [2].
The results of studies showed that PQ mediated neurotoxicity acts mainly via ROS generation with involvement of differential expression patterns for α-syncline in SN, FC and hippocampus of mouse brain. Neuroinflammation took place with or without involvement of microglia activation during PQ treatment and dopaminergic neuronal status changed differentially. Involvement of these differential changes might indicate separate signaling phenomena and different time frames for initiation of neurodegeneration in SN, FC and hippocampus of mouse brain due to PQ treatment [27].
PQ is thought to be transported across the blood brain barrier by the action of a neutral amino acid transporter carrier such as the system L carrier (LAT-1), which normally carries amino acids l-valise and l-phenylalanine, and whose administration has been reported to prevent PQ induced neurotoxicity [27].
Liver and kidney toxicity during PQ exposure: The toxic kinetics of PQ upon ingestion is rapidly but incompletely absorbed. It is rapidly distributed to lung, liver, kidney and muscle [20]. The liver plays a primary role in the metabolism of xenobiotic compounds with biochemical alterations occurring in some toxic conditions [28]. Cytochrome P450 (CYP) is forms especially CYP1A1, CYP1A2 and CYP2E1 have been shown to facilitate formation of ROS during xenobiotic metabolism thereby contributing to oxidative stress induced damage [29]. Direct involvement of CYP-mediated free radical generation has been reported in pesticides [30].
However PQ is very poorly metabolized and is excreted almost unchanged in the urine. Metabolism of PQ has been reported to occur via methylation (monomethyl dipyridone ion) or oxidation (PQ pyridine ion and PQ dipyridone ion) [31].
Additionally, CYP2E1 mediated production of superoxide radicals and hydrogen peroxide in vitro and in transected cultured cells has been documented in previous studies [15]. The results of the study thus demonstrate that PQ differentially regulate hepatic CYP1A1 and CYP1A2 while LPO, glutathione (GSH), CYP2E1, GSTA4 and GSTA3 are modulated in the similar fashions both in the liver and polymorphonuclears (PMNs) [32].
After a few hours, renal clearance declines rapidly in severe poisoning. Thus, the small proportion of PQ that distributes into the deeper compartments is only slowly eliminated by the kidneys over many days to weeks [33]. It is known that, PQ induced apoptosis via elevated cleaved caspase 3 and PUMA in the rat kidneys, which might contribute to the clinical manifestations of PQ induced nephrotoxicity [34].
Lung toxicity during PQ exposure: PQ is not readily absorbed from the gastrointestinal tract, and is even more slowly absorbed across the skin. Upon absorption, independent from the route of exposure, PQ accumulates in the lung, liver, kidney and brain where it exerts its major acute toxicological effects [35].
The lung is the major target organ in PQ poisoning characterized by edema, hemorrhage, interstitial inflammation, and proliferation of bronchial epithelial cells [36] and respiratory failure from lung injury is the most common cause of death. The specific mechanism of lung damage is pulmonary fibrosis, which becomes evident approximately 5 days after PQ ingestion [37]. The main mechanism of PQ’s toxic effects is redoing reaction by ROS, and LPO of cellular membranes is a significant pathway [38]. In addition to redo reaction, inflammatory reaction has been reported as a main mechanism of tissue injury [39]. It is known that under oxidative stress lung cells release inflammatory mediators and cytokines/chemokines, which induces neutrophil recruitment and activation of transcription factors such as nuclear transcription factor be (NF-be) and activator protein-1 (AP-1); thereby augmenting the inflammatory response and tissue damage [40]. Several drugs have been investigated against PQ-induced lung toxicity, but the septic antidote has not been currently founded yet [41]. Severe PQ poisoning produces adult respiratory distress syndrome, pulmonary hypertension, edema and progressive lung broses. Free radicals are known to play a crucial role in PQ-induced lung toxicity [40, 42]. It has been reported that the toxicity is related to redox cycling of an iron PQ complex, which in turn catalyzes the formation of ROS with the ultimate progression of LPO [43].
Pulmonary broses is a progressive and fatal disease with poor prognosis. The etiology of pulmonary broses is still obscure, but it has been accepted that ROS is critically implicated in the development of this chronic disease. PQ causes lung injury that is worsened by increasing the O2 concentration in inspired air [44]. The results of study quercetin administration on PQ toxicity were shown to protect the lung from PQ-induced oxidative stress and the resulting pulmonary injury. A single dose of PQ elevated pulmonary MDA levels significantly when determined 24 h later. These results demonstrated that quercetin (kind of falconoid) administration to rats effectively inhibits the development of PQ-induced pulmonary injury most probably via its antioxidant activity [5, 45]. The pulmonary toxicity caused by PQ is assumed to have a connection with the activation of neutrophils [46]. Furthermore, various inflammatory mediators including TNF-α have been expected to be increased in the lung during PQ toxicity [47]. It is known that TNF-α triggers the synthesis of leukotrienes and prostaglandin E (E2) which then stimulate the infiltration of polymorph nuclear leukocytes into the lungs and cause lung injury [48].
Parquat induced apoptosis: Apoptosis, or programmed cell death (also referred to as an ‘orderly cell deletion’), is a genetically controlled mechanism involved in development, maturation and homeostasis [49]. The term ‘apoptosis’ is often used interchangeably with ‘programmed cell death’. Apoptosis may be induced by stimuli as diverse as hyperthermia, growth factor withdrawal, chemotherapeutic agents, irradiation, cytokines and oxidative stress [50]. Alternatively, low-level oxidative stress is known to activate kinas cascades and transcription factors, such as activator protein-1 (AP-1), hypoxia inducible factor-1α (HIF-1α), and nuclear factor-nab (NF-nab) [51]. Pesticide-induced redo signaling has been demonstrated to mediate many of the toxicological effects of these chemicals. Exposure to a wide variety of pesticides induces oxidative stress reflected as accumulation of ROS, LPO and DNA damage [52]. PQ has been largely demonstrated to induce cell death in a variety of cell types and tissues [53].
PQ induced apoptosis has been demonstrated to involve the intrinsic mitochondrial pathway via activation of back [54] and activation of SAPK [55], consequently, over expression of Bcl-2 protects against PQ induced cell death [56]. Interestingly, PQ induced cytotoxicity has also been suggested to be mediated via the activation of the fast extrinsic pathway of apoptosis [57]. However the previous study showed that after the intragastric administration of PQ, the apoptotic rate and expression of bax protein of lung increased, the expression of Bcl-2 protein decreased, and the Bcl-2/bax ratio was significantly decreased at the same time points compared to the sham group. After early lysine acetylsalicylate (LAS) treatment, the apoptotic rate and bax expression of lung decreased, the Bcl-2 expression increased, and the Bcl-2/bax ratio increased, compared to the PQ group rats [58].
Antioxidant therapy: Accordingly, studies have been performed with different natural substances possessing antioxidant properties to investigate their possible protective effects in PQ induced tissue damage [59]. Importantly the results demonstrate the protective effect of vitamins C and E against PQ toxicity induced in histological damage tissues. These investigations may be attributed to the ability of vitamins C and E to scavenge free radicals resulted from PQ stress [60].
Ascorbic acid, vitamin C, a water soluble vitamin, is effective in scavenging free radicals, including hydroxyl radicals, aqueous proxy radicals and superoxide anions [61]. Ascorbic acid acts as two electron reducing agent and confers protection by contributing an electron to reduce free radicals, thus neutralizing these compounds in the extra cellular aqueous environment prior to their reaction with biological molecules [58]. Moreover, the antioxidant potential of ascorbic acid is not only attributed to its ability to quench ROS, but also to its ability to regenerate other small antioxidant molecules, such as α-tocopherol, glutathione and β carotene [62]. The role of vitamin E in PQ toxicity was demonstrated in several studies where deficiency of vitamin E potentiated the development of acute PQ toxicity in animals. It was shown that vitamin E deficiency shortened and decreased survival, worsened histological lung damage in rats [63]. Moreover, the potentiating of acute PQ toxicity by vitamin E deficiency was reversed by administration of vitamin E [64]. Although the mechanism by which vitamin E protects against PQ toxicity is not understood, it may be attributed to its antioxidant properties in preventing LPO or inhibiting the generation of superoxide anion and its toxicity [59]. Results from another study demonstrated that PQ was able to induce the formation of micronuclei, commonly used to assess chromosomal damage, in polychromatic erythrocytes (PCE), both in the bone marrow and in the peripheral blood of mice, a treatment effect attributed to the generation of ROS [65]. Administration of melatonin to these mice conferred protection against the PQ induced micronuclei and this effect was attributed to the antioxidant properties of the pineal secretary product [66].
The administration of desferoxamine (DFO) by continuous intravenous infusion to vitamin E deficient rats significantly reduced mortality produced by PQ [67]. It has been shown that DFO can exert its protective effects, not only by inhibiting the PQ induced generation of hydroxyl radicals, but also by blocking the uptake of PQ by the alveolar type II cells [68]. Administration of more lipophilic chelating agents, such as hydroxypyridin-4-one (CP 51), also increased the survival of PQ challenged rats with a normal vitamin E status. Moreover, the protective effect of CP51 was also demonstrated in vitro experiments where CP51 prevented the PQ induced lyses of alveolar type II cells [68]. Although experimentation with iron cheaters against PQ induced toxicity seems promising, iron chelating therapy in human poisoning remains to be established. N-acetylcysteine (NAC), the acetylated variant of the amino acid L-cytokine, is an excellent source of sulfhydryl (SH) groups. NAC is converted in the body into metabolites capable of stimulating GSH synthesis, promoting detoxification and acting directly as free radical scavenger [69].
In another study, the administration of NAC to PQ challenged animals delayed the PQ induced release of chemo attractants for neutrophils in the broncheoalveolar ravage fluid and significantly reduced the infiltration of inflammatory cells suggesting that NAC can confer its protective effect by delaying inflammation [70] (Table 1).
In addition to its established anti-inflammatory mechanism of inhibiting cyclo-oxygenase, salicylic acid (SA) has a variety of antioxidant effects. It can scavenge hydroxyl radicals and inhibit their production through the Fenton reaction [71]. SA can reduce oxidative stress [72]. There are several studies, the administration flavonoides on modulation of PQ toxicity [6, 73].
| Study | Species | Effective antioxidants | Toxicity model | Results |
|---|---|---|---|---|
|
Harrison et al. [ | Mouse | Ascorbic acid (Vitamin C) | Lung toxicity | ↑ Oxidative stress in lung, F2-isoprostanes, ↓ Sodium dependent vitamin C transporter 2 (SVCT2) |
|
Hanada et al. [ | Cell line | Vitamin E (α-tocophrol) | Gene expression | ↑ Chromosomal damage, ↑ catalase |
|
Singhal et al. [ | Mouse | Melathonin | Gene expression | ↑ Lipid peroxidation, ↑ degenerating neurons, ↑ nitrite content, ↑ mRNA expressionsCYP2E1 and GSTA4-4 |
|
Kielar et al. [ | Cell line | Iron chelators | Cytotoxicity | ↑ Oxidative stress, ↑ mRNA level |
|
Kumar et al. [ | Rat | Glutathione, trace elements | Neurotoxicity, gene expression | ↑ Oxidative stress, ↑ NADPH oxidase, ↑ cytochrome C release, caspases-9 and -3 and CD11b expression |
|
Mitsopoulos et al. [ | Cell line | N-acetylcysteine | Cytotoxicity, gene expression | ↑ Cytotoxicity in A549 cells, ↓ intracellular glutathione content, ↑ ROS levels, ↑ mitochondrial membrane potential, ↑ cellular gene expression, inflammatory cytokine release |
|
Park et al. [ | Rat | Flavonoides | Lung toxicity | ↑ Pulmonary MDA level and HO-1 expression and NO, ↑ fibroblast distribution and collagen deposition in the lungs, ↑ total oxyradical scavenging capheme oxygenase-1acity (TOSC) |
|
Somayajulu-Nitu et al. [ | Rat | Ubiquinone (Coenzyme Q) | Neurotoxicity | ↑ Oxidative stress markers, ↓ loss of approximately 65% of dopamine neurons |
|
Kumar et al. [ | Rat | Gene expression | ↑ Neurodegeneration, ↑ oxidative stress, ↑ expression of genes | |
|
Ahmad et al. [ | Rat | Liver and gene expression | ↑ Expression and catalytic activity of CYP2E1 and GSTA4-4, ↑ expression of GSTA3-3 and GSTA1-1 | |
|
Singhal et al. [ | Mouse | Flavonoides | Gene expression | ↑ lipid peroxidation, ↑ degenerating neurons, ↑ nitrite content, ↑ mRNA expressions of cytochrome CYP2E1 and GSTA4-4 |
|
Zega et al. [ | P. mammillata | l-Ascorbic acid | Neurotoxicity | ↑ Dopamine content in anterior CNS, oxidative stress is involved |
|
Sun et al. [ | Rat | Flavonoides | Lung injury | levels of MDA, ↓ NF-β and TNF-α, ↑ the activities of GSH-Pox and SOD |
|
Podder et al. [ | Cell line | Flavonoides | Gene expression | ↑ Oxidative stress, ↓ LDH, ↓ anti-oxidant related genes, including Nrf2, NQO1 and HO-1 |
The list of some studies in parquet toxicity
Discussion
In summary the results suggest that antioxidant materials effectively prevent the pulmonary injury induced by PQ treatment in rats and humans. Instillation of PQ resulted in reduction of antioxidant capacity, elevation of inflammatory response, collagen accumulation and fibrotic changes in organisms. There are various (effective) antioxidants such as vitamins E and C, quercetin, NAC, melatonin in PQ toxicity, therefore, is in need of a new therapeutic approach. The ideal antioxidant treatment for PQ poisoning should involve intracellular and extracellular protection against superoxide anion, hydrogen peroxide, hydroxyl radical and membrane lipid peroxidation. Many remains to be learned about the role of intracellular and extracellular antioxidants in PQ toxicity Thus, further research is warranted to determine the effects of a combination of antioxidants in the treatment of PQ poisoning and these studies may be a potential aid to manage patients suffering from PQ intoxication. Although a large amount of toxicological studies have been performed to understand the biological basis of the cellular response to PQ, there is still a lack of evidence for a clear mechanistic explanation of these effects.