1. Background
Rheumatoid arthritis (RA) is a common systemic autoimmune disease characterized by chronic inflammation and irreversible destruction of joints and bones (1). The disease has a prevalence of 0.5% and 1% in European and North American populations, respectively, and 0.1% in North Africa (2). It is four times more prevalent in women than men due to sex hormones and genetic predisposition (3). Several studies found that comorbidities differ between genders (3, 4). Moreover, the published data concluded different clinical aspects, including age, biological assessment, radiological damage, and comorbidities between genders (5).
Age at RA onset could be considered a criterion of poor prognosis frequently found in the literature, which explains the importance of studies and research about the effect of age-at-onset on RA development (6). As accepted, late-onset RA (LORA) usually begins after 50 to 65 years, while young-onset RA (YORA) develops between 30 to 45 years. Besides, YORA is characterized by a high rate of remission, a lower frequency of radiographic progression and functional score, a higher rate of anti-CCP and RF than LORA (7).
2. Objectives
This study aimed to investigate the gender and age at RA onset differences in the western Algerian population and their effects on clinical features and medical management of Algerian RA patients.
3. Methods
3.1. Population
This cross-sectional study was done at the level of Internal Medicine in partnership with the Functional Rehabilitation Departments of the University Hospital of Sidi-bel-Abbes region from 2016 to 2019 on the records of over 306 RA patients diagnosed according to ACR 1987 criteria. We recorded the demographic characteristics, such as sex and age, and clinical features, including disease duration, Disease Activity Score 28 (DAS28, running from 0 to 10), radiologic progression, laboratory assessment, and medication. We aimed to make a comparative study of gender and age-at-onset in RA. Besides, LORA was defined as a disease onset at 51 years of age or older.
3.2. Statistical Analysis
Our results were presented as frequencies and percentages for categorical variables using Pearson chi-square test (χ2) and means and standard deviations for continuous variables using the independent sample t test. Values are expressed as numbers (percentages) or mean ± standard deviation. Anti-CCP and rheumatoid factor (RF) ratios were compared according to age-at-onset using the correlation test. All data were processed and analyzed via SPSS 22.0 (Statistical Package for the Social Sciences, IBM Corporation; Chicago, IL, August 2013). The level of significance was set at < 5%.
4. Results
4.1. Patient Characteristics
We enrolled 306 RA patients (85% women) with a mean age of 52.47±12.14.
4.2. Gender Difference
The sample included 260 women and 46 men of comparable age-at-onset of RA (52.58 ± 2.23 and 53.65 ± 11.68 years, respectively; P = 0.58), disease duration (4.15 ± 3.93 and 4.56 ± 4.06, respectively; P = 0.52), and DAS28 (4.52 ± 1.22 and 4.63 ± 1.23, respectively; P = 0.6). Hand joints were the most reported sites of joint disorder in both genders (57.5% and 10.8%, respectively; P = 0.59). Besides, 21.2% of women had erosive RA (P = 0.87). We did not find any significant relationship between laboratory data (CRP, ESR, anti-CCP, and RF), treatment, and gender. Women presented anemia more frequently than men (21.9% vs. 1%; P = 0.004) (Table 1).
| Women | Men | P Value | |
|---|---|---|---|
| Age-at-onset | 52.588 ± 2.2359 | 53.652 ± 11.6871 | 0.585 |
| Disease duration | 4.158 ± 3.9341 | 4.565 ± 4.0642 | 0.52 |
| Region | 0.851 | ||
| Rural | 70 (22.9) | 13 (4.2) | |
| Urban | 190 (62.1) | 33 (10.8) | |
| Radiologic joint damage | |||
| Hands | 176 (57.5) | 33 (10.8) | 0.59 |
| Wrists | 161 (52.6) | 30 (9.8) | 0.67 |
| Knees | 146 (47.6) | 24 (7.8) | 0.61 |
| Elbows | 95 (31) | 21 (6.9) | 0.24 |
| Shoulders | 93 (30.4) | 15 (4.9) | 0.68 |
| Feet | 77 (25.2) | 17 (5.6) | 0.32 |
| Ankle | 35 (11.4) | 10 (3.3) | 0.14 |
| Erosion | 65 (21.2) | 12 (3.9) | 0.87 |
| DAS28 | 4.5286 ± 1.2290 | 4.632 ± 1.2390 | 0.6 |
| ESR | 43.446 ± 24.7641 | 43.478 ± 24.9798 | 0.25 |
| CRP | 19.2303 ± 30.8062 | 13.9391 ± 12.4531 | 0.99 |
| Anti-CCP positivity | 208 (68) | 38 (12.4) | 0.68 |
| RF positivity | 209 (68.3) | 40 (13.1) | 0.29 |
| Anemia | 67 (21.9) | 3 (1) | 0.004 |
| DMARDs | |||
| Methotrexate | 215 (70.3) | 34 (11.1) | 0.16 |
| Leflunomide | 40 (13.1) | 8 (2.6) | 0.73 |
Clinical, Radiologic, and Laboratory Data in Rheumatoid Arthritis Patients Based on Gender
Table 2 illustrates the comorbidity profile against gender in RA. As observed, most of the men were smokers (P < 0.001). Also, 13.7% and 36.6% of patients (women) suffered from type 2 diabetes and hypertension, respectively, with a significant correlation (P = 0.035 and P = 0.003, respectively). Moreover, 5.6% of patients had lung disorders, including 4.9% of men (P < 0.001). A significant correlation was found between thyroid disorders and female gender (P = 0.05).
| Women | Men | P Value | |
|---|---|---|---|
| Smoking | 0 (0) | 31 (10.1) | < 0.001 |
| Type 2 diabetes | 42 (13.7) | 2 (0.7) | 0.035 |
| Hypertension | 112 (36.6) | 9 (2.9) | 0.003 |
| Solid neoplasia | 3 (1) | 0 (0) | 0.46 |
| Osteoporosis | 22 (7.2) | 1 (0.3) | 0.136 |
| Thyroid disorders | 20 (6.5) | 0 (0) | 0.05 |
| Stomach ulcers | 7 (2.3) | 0 (0) | 0.26 |
| Pulmonary disease | 2 (0.7) | 15 (4.9) | < 0.001 |
| Scleroderma | 9 (2.9) | 0 (0) | 0.20 |
Comorbidity Profile Against Gender in Rheumatoid Arthritis Patients
4.3. Age Difference
More than half of our patients (60.5%) were in the LORA group (51% women and 9.5% men; P = 0.69). The mean disease duration was 3.82 ± 3.23 in YORA against 4.47 ± 4.34 years in LORA (P = 0.16). A significant relationship was found between LORA and knee damage (P = 0.008) but not with erosion, DAS28, and ESR. Most of the patients (44.8%) in the LORA group presented positive anti-CCP and RF (P = 0.001 and P < 0.001, respectively) (Table 3). Concerning comorbidities, we noticed a dominance of hypertension, osteoporosis, and scleroderma in the LORA group (P < 0.001, P = 0.007, and P = 0.014, respectively) (Table 4).
| YORA (≤ 50 Years) | LORA (≥ 51 Years) | P Value | |
|---|---|---|---|
| Age-at-onset | 40.529 ± 7.919 | 60.741 ± 6.408 | — |
| Disease duration | 3.826 ± 3.2318 | 4.476 ± 4.3453 | 0.16 |
| Area | 0.756 | ||
| Rural | 34 (11.1) | 49 (16) | |
| Urban | 87 (28.4) | 136 (44.4) | |
| Joint damage | |||
| Hands | 84 (27.5) | 125 (40.8) | 0.73 |
| Wrists | 80 (26.1) | 111 (36.3) | 0.28 |
| Knees | 56 (18.3) | 114 (37.3) | 0.008 |
| Elbows | 47 (15.4) | 69 (22.5) | 0.78 |
| Shoulders | 45 (14.7) | 63 (20.6) | 0.57 |
| Feet | 32 (10.5) | 62 (20.3) | 0.19 |
| Ankle | 15 (9.4) | 30 (9.8) | 0.35 |
| Erosion | 37 (12.1) | 40 (13.1) | 0.078 |
| DAS28 | 4,5534 ± 1,2801 | 4,5380 ± 1,1979 | 0.91 |
| ESR | 42,5619 ± 25,1017 | 44,0324 ± 24,5772 | 0.61 |
| CRP | 18,5157 ± 38,0237 | 18,3819 ± 20,8997 | 0.96 |
| Anti-CCP positivity | 109 (35.6) | 137 (44.8) | 0.001 |
| RF positivity | 112 (36.6) | 137 (44.8) | <0.001 |
| Anemia | 26 (8.5) | 44 (14.4) | 0.64 |
| DMARDs | |||
| Methotrexate | 103 (33.7) | 146 (47.7) | 0.17 |
| Leflunomide | 20 (6.5) | 28 (9.2) | 0.74 |
Clinical, Radiologic and Laboratory Data in Rheumatoid Arthritis Patients Based on Age-at-onset
| YORA (≤ 50 Years) | LORA (≥ 51 Years) | P Value | |
|---|---|---|---|
| Smoking | 13 (4.2) | 18 (5.9) | 0.774 |
| Type 2 diabetes | 12 (3.9) | 32 (10.5) | 0.072 |
| Hypertension | 27 (8.8) | 94 (30.7) | < 0.001 |
| Solid neoplasia | 1 (0.3) | 2 (0.7) | 0.825 |
| Osteoporosis | 3 (1) | 20 (6.5) | 0.007 |
| Thyroid disorders | 8 (2.6) | 12 (3.9) | 0.96 |
| Stomach ulcers | 2 (0.7) | 5 (1.6) | 0.548 |
| Pulmonary disease | 6 (2) | 11 (3.6) | 0.712 |
| Scleroderma | 0 (0) | 9 (2.9) | 0.014 |
Comorbidity Profile of Rheumatoid Arthritis Patients Against Age-at-onset
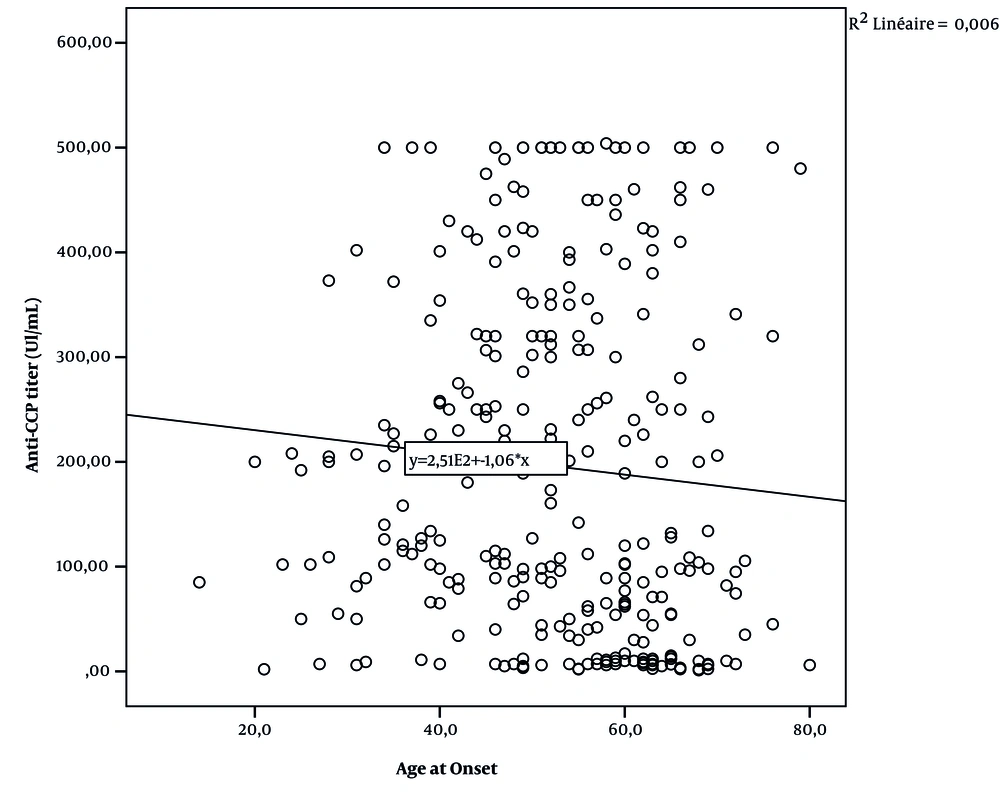
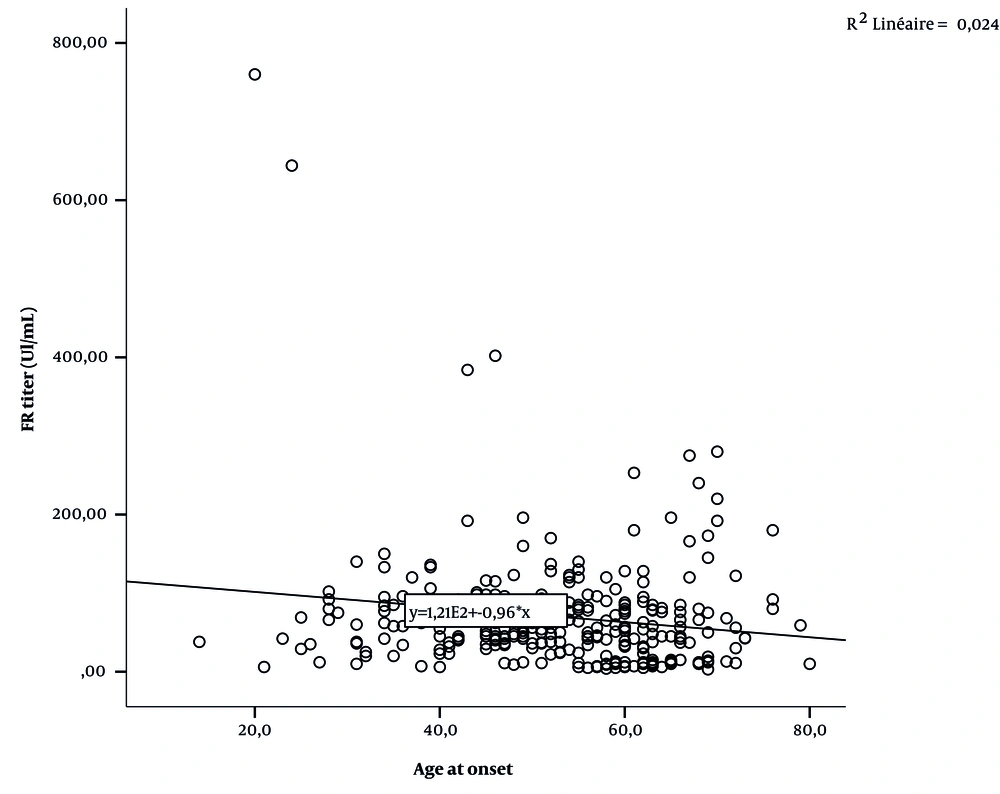
Figures 1 and 2 and Table 5 show the correlation between serological rates (anti-CCP and RF titer) and age-at-onset.
| Variables | Age-at-onset (y) | |
|---|---|---|
| r (Correlation Coefficient) | p | |
| Anti-CCP | -0.77 | 0.18 |
| RF | -0.155 | 0.006 |
Correlation Between Age-at-onset and Serological Parameters
5. Discussion
To the best of our knowledge, the current study is the first that examines the profile of rheumatoid arthritis against gender and age-at-onset in the western Algerian population. This study aimed to evaluate the impacts of gender and age on clinical characteristics, medical management, and comorbidities of rheumatoid arthritis patients.
5.1. Gender Difference
The clinical features of RA patients vary according to sex (8). Our study demonstrated that Algerian RA women suffered from comorbidities more than men. Cross-sectional studies in Latin-American countries reported a clear female predominance (3). Ouali et al. (9) reported that most patients were women, which concords with our results and could be explained by the impact of sex hormones on the immune system (10). In the present study, we found no significant relationship between clinical variables (mean age, disease duration, and DAS28) and gender of RA patients, as confirmed by several studies (4, 11, 12). It also agrees with Barragán-Martínez et al. (3), Ahlmén et al. (13), and Coffey et al. (14) studies.
Anti-CCP and RF can be predictors of rheumatoid arthritis activity (15). Our results add to several authors' findings (4, 9, 14, 16, 17), highlighting that anti-CCP or RF titer did not differ between female and male RA patients. Similar to our prior results, erosions were not associated with gender groups (13, 14).
Anemia is a common comorbidity associated with RA (18). Agrawal et al. (19) illustrated that RA women suffered from anemia more than RA men. Our cohort found a significant correlation between anemia and female sex (P = 0.004). Besides, most RA men were smokers (P < 0.001). Our results and those of Uhlig et al. (20), Manfredsdottir et al. (21), and Ruiz-Esquide et Sanmartí (22) emphasize the relationship between smoking and RA risk in men.
Dougados et al. (23) confirmed the strong association of some comorbidities with rheumatoid arthritis. According to several data, there is a high impact of sex on RA comorbidities (24). We reported a significant gender difference according to some comorbidities. Sex hormones have a significant impact on the development of type 2 diabetes mellitus (25, 26). Accordingly, previous studies reported a significant correlation between female sex and type 2 diabetes in RA patients (4, 11), and our results confirm this association (P = 0.0035).
Medication, inflammations, and oxidative stress were the most risk factors of hypertension prevalence in RA patients (27, 28). Aurrecoechea et al. (4, 11) did not find any correlation between gender and hypertension. Other studies disagreed with this investigation (3, 29), similar to our survey (P = 0.003). On the other hand, many studies confirmed the association between RA and autoimmune thyroid disease (AITD) (30). It was reported that women are affected more than men by AITD (31), with an incidence of 4.4% (× 1,000,000) in the USA (32). Barragán-Martínez (3) affirmed that RA women are most affected by AITD, with a significant association (P = 0.005).
Our results illustrated a significant association of pulmonary disease with the men group (P < 0.001). Aurrecoechea et al. (4, 11) attempted to explain this association. Nevertheless, other investigators demonstrated pulmonary disease prevalence among males due to a genetic predisposition (33, 34).
5.2. Age Difference
We aimed to evaluate the impact of age-at-onset on RA patients' clinical and medical features. The RA incidence is relatively high in elderly-onset RA (35) due to the immunosenescence phenomenon associated with age (36). Clinical and medical management of RA differs between elderly and young subjects with RA (37), including serology, medication, and comorbidities (38-41). Our results are consistent with previous findings, but we did not observe any significant differences in disease duration (7, 42), articular erosion, and DAS28 (40, 41, 43) based on age-at-onset.
Anti-CCP was more often positive in the young-onset RA (YORA) than in the late-onset RA (LORA) groups (44), with a higher frequency of the PTPN22 T-variant (45). Our study found evidence of an association between serology status (anti-CCP and RF) and age-at-onset (P = 0.001, P < 0.001). Similarly, the results of other investigations confirmed a significant association between the anti-CCP titer and age-at-onset in RA patients (7, 9, 46). Tan et al., Chen et al., and Sparks et al. (40, 41, 47) illustrated a significant relationship between positive RF and age. However, García de Veas Silva et al. and González-Febles et al. (48, 49) did not observe any significant relationship between seropositive RA and age.
It was noted that comorbidities were more common in older subjects with a higher prevalence (50). The LORA group presented a significant association with hypertension and osteoporosis (P < 0.001) (40, 51), which is similar to the current investigation. Furthermore, the median age at diagnosis was 50 - 58 years in scleroderma (52, 53). Our study confirmed the significant relationship between LORA and scleroderma (P = 0.014).
Since this is a retrospective study, we were unable to conclude the impact of gender and age-at-onset on disease severity, RA outcome, and DMARDs choice.
5.3. Conclusions
Clinical, medical, and biological features of RA patients did not differ between genders. However, the comorbidity profile was different between women and men. Anti-CCP and RF were higher in the YORA group. Nevertheless, patients with LORA presented some comorbidities, with a significant impact. Further studies are required to prove the effects of gender and age-at-onset on specific outcomes in RA.