1. Background
Stroke is one of the most important causes of severe long-term disability worldwide. Each year, 15 million people worldwide have a stroke. During ischemic stroke, molecular and cellular processes lead to destruction of neurons, glial cells, and capillary blood vessels. Cytotoxicity, oxidative stress, lipid peroxidation, inflammation, blood-brain barrier breakdown, and cerebral edema are some of these processes [1, 2].
Increased levels of reactive oxygen species (ROS) and reactive nitrogen species (RNS) produced from oxidative stress induce mitochondrial dysfunction, calcium overload, inflammation, and reperfusion injury [3]. Excessive ROS formation can deplete endogenous antioxidant and cause cellular damage [4]. Nitric oxide (NO) at low concentrations serves as a gaseous neurotransmitter in many physiological processes but at high concentrations causes pathological conditions. Excessive NO produced after ischemia and reperfusion reacts with superoxide to form proxy nitrite and other forms of RNS. Further interaction of proxy nitrite with lipids, DNA, and proteins may cause harmful pathophysiological events [5, 6].
Induction of ischemia for some minutes in the hippocampal region of the brain can lead to learning and behavioral deficits. Cerebral ischemia has been also reported to impair motor coordination and balance [7-9].
Recently, a number of medicinal plants have been shown to protect neurons against ischemic stroke [10]. Matricaria chamomilla is native to the Mediterranean region but is now widely disturbed worldwide. In Iranian traditional medicine, M. chamomilla is used as an anti-fever, antispasmodic, and sedative agent [11]. The aim of the current study was to examine the effect of ethyl alcohol M. chamomilla extract on cerebral ischemia-induced motor dysfunctions in rats.
2. Methods
2.1. Preparation of Matricaria chamomilla Extract
In this experimental study, the dried plant materials were finely pulverized and then 50 g of the resulting powder was mixed with 500 mL of 70% ethyl alcohol. After 48 hours, the mixture was filtered through a Buchner funnel and then the resulting filtrate was concentrated using a rotary evaporator and dried in an oven at 40°C. The antioxidant capacity of Matricaria chamomilla extract was determined using DPPH free radical scavenging assay [12]. Total phenolic content of Matricaria chamomilla extract was determined using the Folin–Ciocalteu colorimetric assay [13], and the total flavonoid and flavonol content of Matricaria chamomilla extract was determined using colorimetric assay [14].
2.2. Experimental Animal and Grouping
Forty two healthy male Wistar rats weighing 250 - 300 g were housed under controlled conditions (12:12h light/dark cycle at 23 ± 2°C) with free access to water and standard rodent pellet. All experiments were conducted according to the guide for the care and use of laboratory animals. The rats were randomly assigned to six groups of seven each as follows:
- Control group: Rats receiving distilled water;
- Sham group: Rats underwent sham operation without carotid occlusion;
- Ischemic group: Rats undergoing ischemia and receiving distilled water alone;
- Extract-treated groups: Rats undergoing ischemia and administered with 50, 100 and 200 mg/kg/day of Matricaria chamomilla extract intraperitoneally for 15 days.
Induction of ischemia:
First, the rats were anesthetized and then a midline incision was made on the anterior region of the neck to expose carotid sheath, and common carotid arteries were visualized and separated meticulously from vagal nerve. Acute ischemic stroke was induced by occlusion of the common carotid arteries for 60 minutes. Blood flow was then restored. During the surgical procedure, the rats’ bodies temperature was kept at 37°C using an infrared heat lamp [13].
Rotarod test was performed after 15 days of treatment. After Rotarod test, blood samples of the rats’ hearts were collected and their brains were removed under deep anesthesia. The serum of the blood samples was isolated by centrifugation and stored at -20°C untilbiochemical analysis. After removal of the brain, hippocampus and cerebral cortex were separated on an ice and used for biochemical analysis
2.3. Evaluation of Motor Coordination and Balance
Motor function and balance were determined using Rotarod apparatus. The speed of apparatus was 10 rpm and the maximum trial length was 300 seconds. Before conducting the test, the rats were trained to walk on the rotating rod of Rotarod. Then, each rat was placed on the rotating rod and the length of the rats’ balance was recorded to represent resistance time [14].
2.4. Measurement of Brain Antioxidant Capacity
The brain antioxidant capacity was measured by FRAP assay. The FRAP reagent was prepared by mixing acetate buffer (25 mL), TPTZ solution (2.5 mL), and FeCl3, and 6H2O solution (2.5 mL). Homogenized brain sample (in 2.5% cold KCl solutions) was centrifuged and supernatant (50 µL) was mixed with 1.5 mL of fresh FRAP solution. After 10 minutes at 37, the absorbance of the mixture was recorded at 590 nm wavelength [15].
2.5. Measurement of Serum Antioxidant Capacity
Three different solutions were used to measure the serum antioxidant capacity: Solution 1: 1.5 ml of sodium acetate and 8 ml of concentrated acetic acid diluted to 5000 mL with distilled water; solution 2: 270 mg of ferric chloride diluted to a 50 mL volume with distilled water; solution 3: 47 mg of treeazin dissolved in 40 mL of HCl. Working solution was prepared by mixing 10 mL of the solution 1, 1 mL of the solution 2, and 1 mL of the solution 3. Twenty five microl of the serum samples was added to 1.5 mL of the working solution. The reaction mixture was kept at 37°C for 10 minute and then the absorbance was read at 593 nm wave length [15].
2.6. Measurement of Serum Malondialdehyde
Serum (50 µL) was mixed with 50 µL of BHT (0.05% in 95% ethanol), 400 µL of H3PO4 (0.44. M), and 100 µL of TBA and heated at 100°C for 1 hour. After cooling at 0 for 5 minutes, butanol was added and centrifuged (14,000 rpm for 5 minutes). Then, the absorbance of the supernatant was read at 532 nm wave length [15].
2.7. Measurement of Brain Malondialdehyde
Brain tissue (1 g) was homogenized in 10 mL of 2.5% cold KCl solutions. One mL of the homogenate was transferred into a 20-mL tube and incubated at 37°C for 60 minutes. Afterwards, the suspension was mixed with 1 mL of TCA (5%) and 1 ml of TBA (67%), and centrifuged for 15 minutes at 2000 rpm. The supernatant was transferred into a new tube and incubated for 10 minutes in a boiling water bath. After cooling, the absorbance was read at 535 nm wavelength using spectrophotometer [16].
2.8. Measurement of Serum Nitrate Level
First, the serum samples were deproteinized with zinc sulfate and sodium hydroxide solutions. After centrifugation, the supernatant was mixed with glycine buffer. Cadmium granules were activated by swirling in a CuSO4 solution in glycine-NaOH buffer. Then, activated cadmium granules were added to the deproteinized serum. After continuous stirring for 10 minutes, the resulting solution was transferred to a new tube, mixed with Griess reagent, and kept at room temperature in darkness for 30 minutes. Finally, Griess2 reagent was added and the absorbance was read at 540 nm wavelength [14].
2.9. Statistical Analysis
All data were analyzed using SPSS 16 and expressed as the mean ± standard deviation (SD). One-way ANOVA followed by Tukey’s or Dunnett’s T3 were used to compare the data of different groups. P < 0.05 was considered statically significant.
3. Results
3.1. Effect of Matricaria chamomilla Extract on Motor Coordination and Balance
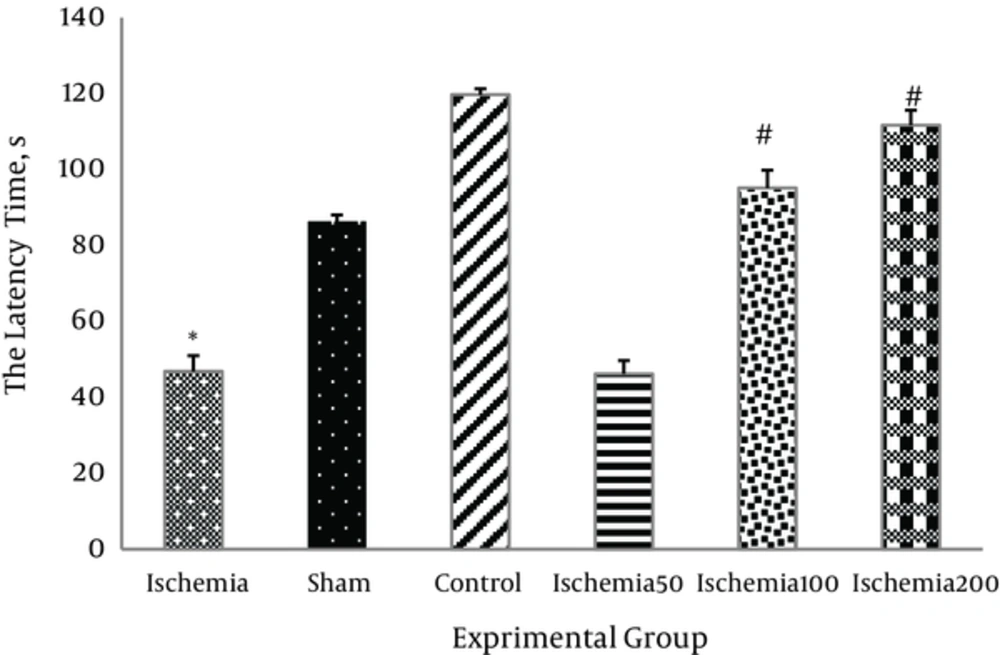
The latency to fall from the rotating rod in the ischemic group was significantly lower than in the control group (P < 0.05). Administration of 200 mg/kg/day Matricaria chamomilla extract at 100 and 200 mg/kg significantly increased motor coordination in the ischemic rats (P < 0.05, Figure 1).
3.2. Effect of Matricaria chamomilla Extract on Brain Antioxidant Capacity
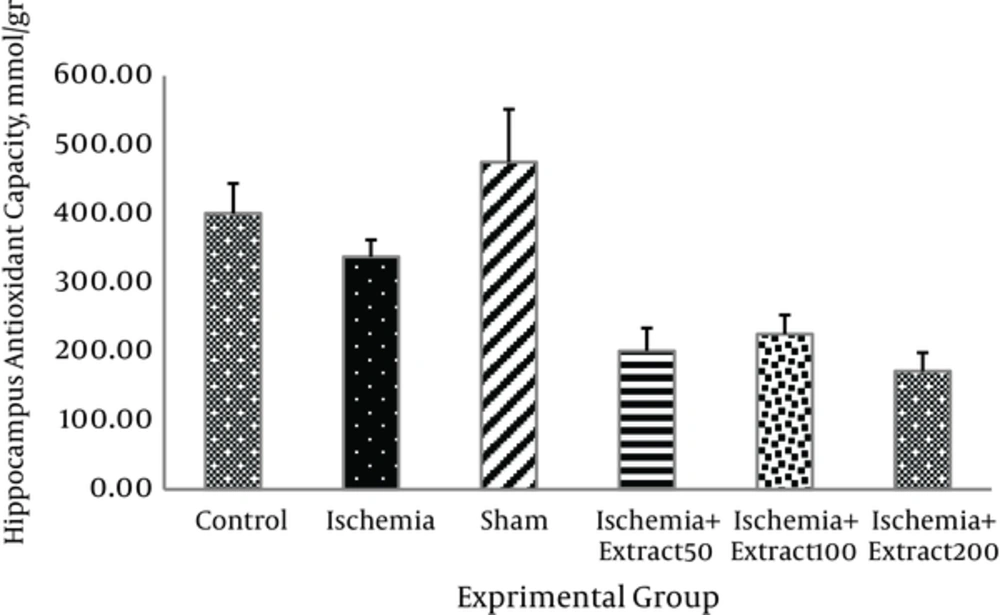
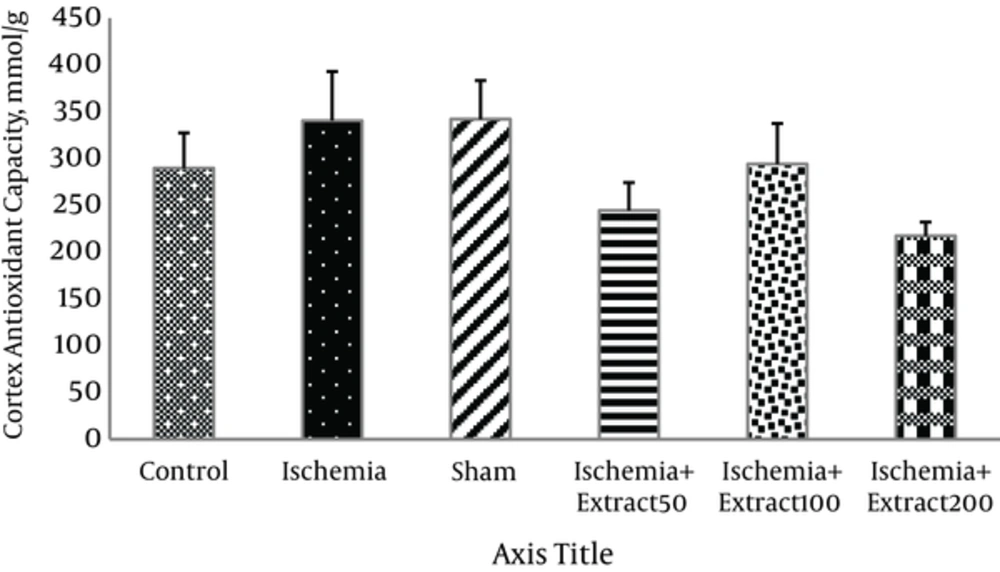
Administration of different doses of Matricaria chamomilla extract had no significant effects on the antioxidant capacity of hippocampus and cortex in the ischemic rats (Figures 2 and 3).
3.3. The Effect of Matricaria chamomilla Extract on Serum Antioxidant Capacity
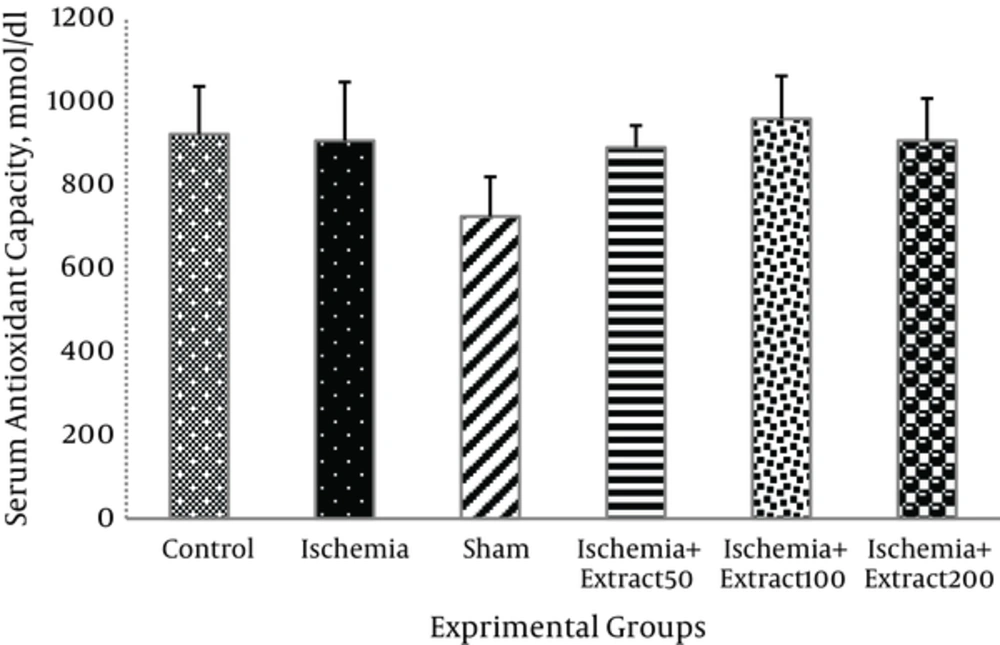
There was no significant difference in the serum antioxidant capacity among the six groups (P > 0.05) (Figure 4).
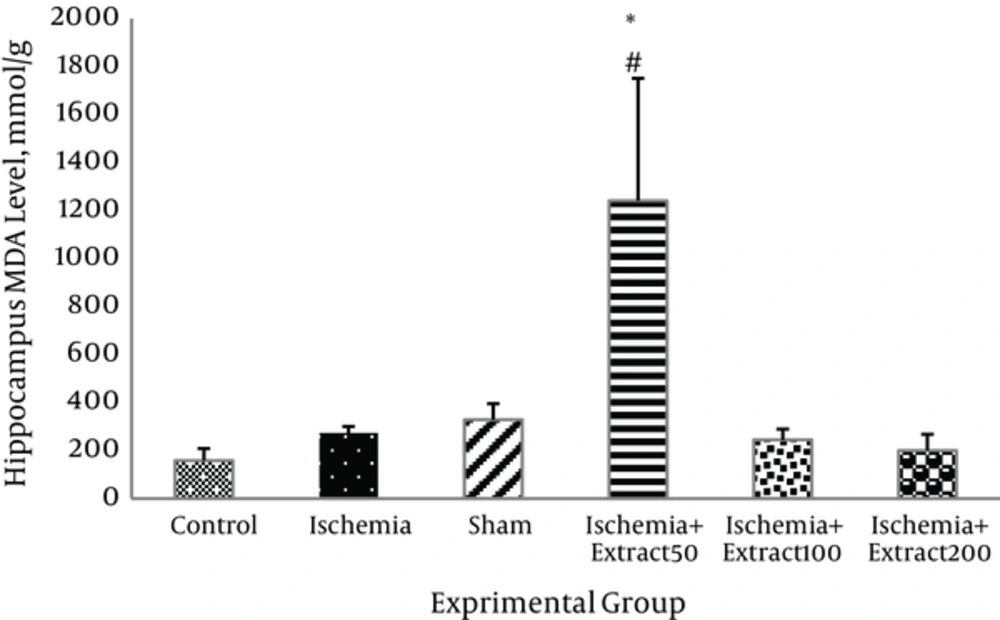
3.4. Effect of Matricaria chamomilla Extract on Brain MDA Level
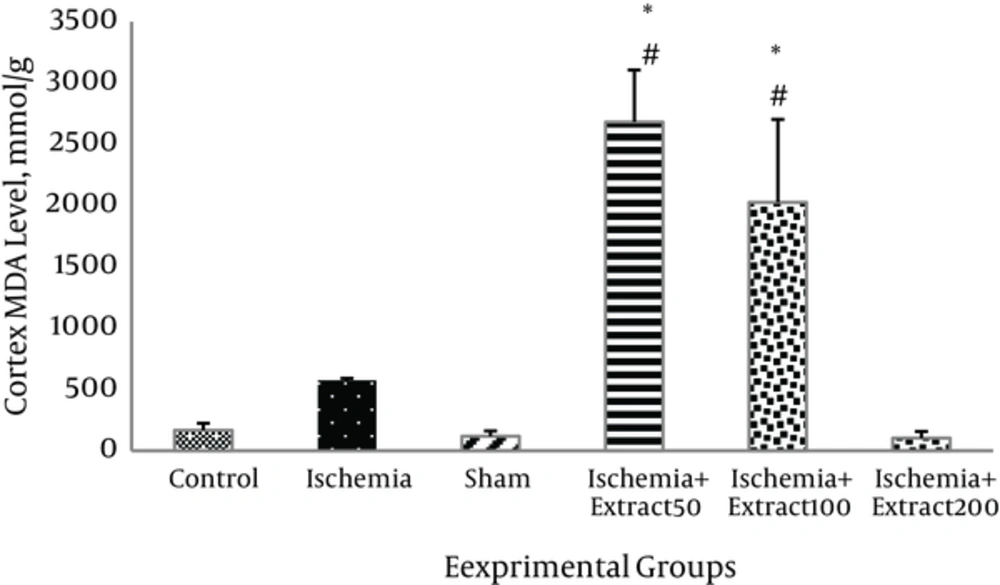
MDA level in the hippocampus of the ischemic rats was slightly and insignificantly higher than in those of the control group. Administration of Matricaria chamomilla extract to the ischemic rats did not reduce the hippocampus MDA levels (P > 0.05).MDA levels in the cortex of 50 mg/kg/day extract treated groups were significantly higher than those of the other groups (P < 0.05, Figure 5). As shown in Figure 6, cerebral ischemia induced an increase in cortex MDA content as compared to the control group; however, this increase was not significant (P > 0.05). Two hundred mg/kg of Matricaria chamomilla extract caused slight decrease in MDA level in the cortex of the ischemic rats (P > 0.05). MDA levels in the cortex of 100 and 50 mg/kg/day extract treated groups were significantly higher than those of the other groups (P < 0.05).
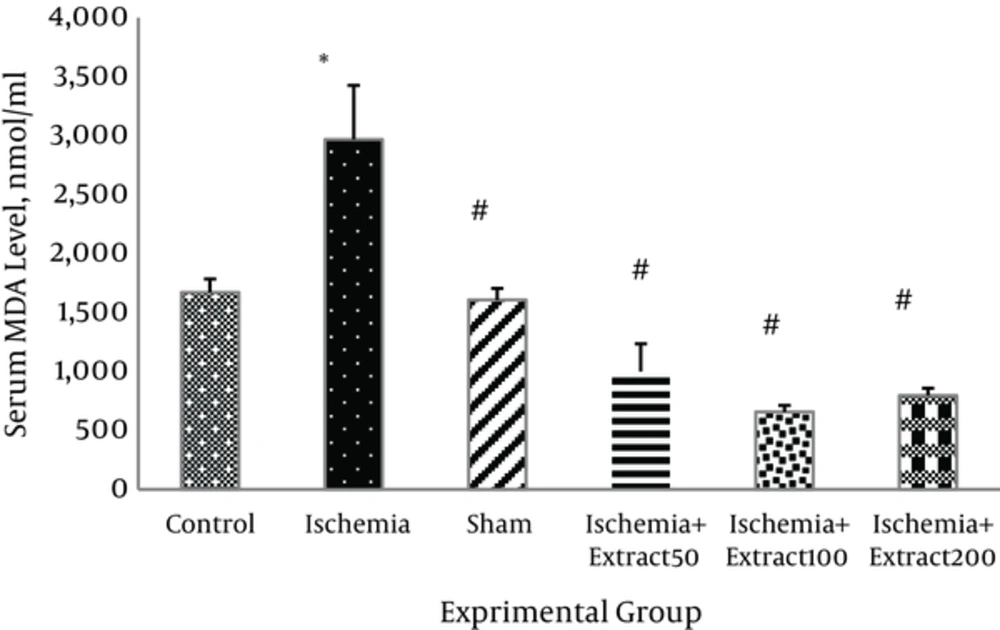
3.5. Effect of Matricaria chamomilla Extract on Serum Malondialdehyde Level
The MDA levels were significantly elevated in the serum of the rats exposed to transient cerebral ischemia (P < 0.01). The extract of Matricaria chamomillain 50, 100, and 200 g/kg/day doses significantly decreased the serum MDA level (P < 0.01) (Figure 7)
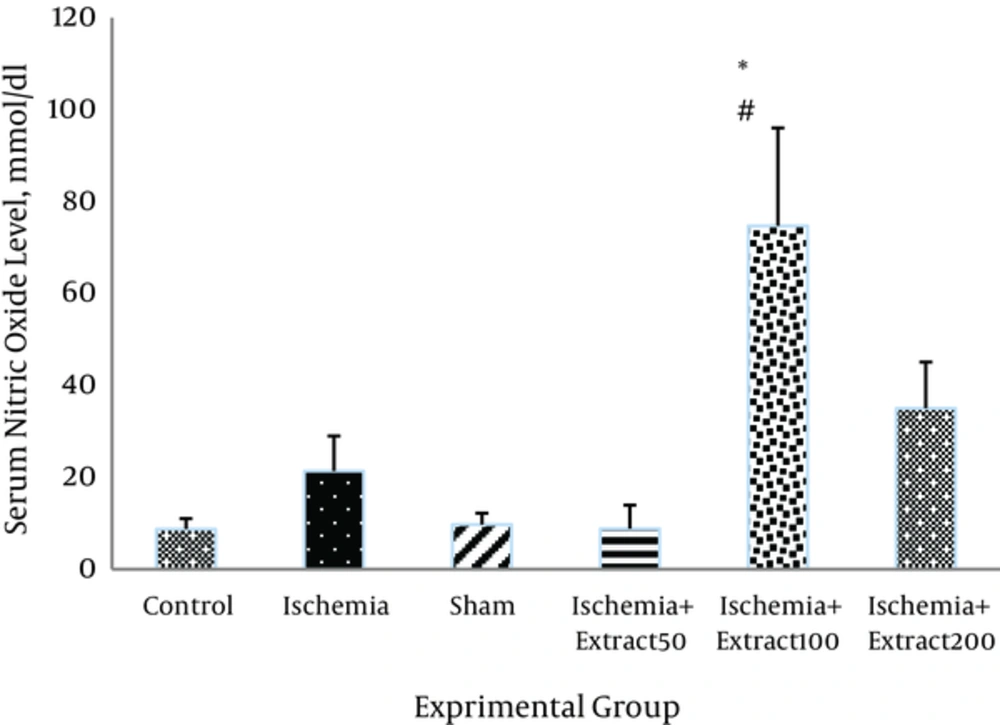
3.6. Effect of Matricaria chamomilla Extract on Serum NO Content
As shown in Figure 8, NO level was elevated following ischemia and reperfusion in the rats but not significantly. Administration of Matricaria chamomilla extract (50 mg/kg) caused a reduction in serum NO level in the ischemic rats; however, this reduction was not statically significant (Figure 8)
3.7. Antioxidant Capacity of Matricaria chamomilla Extract
Table 1 shows the antioxidant capacity of Matricaria chamomilla according to DPPH method. The IC50 of Matricaria chamomilla was 49.2.Total phenolic, flavonoid, and flavonol contents of the Chamomile extracts were 550.654, 234 and 377.998 mg, respectively.
| DPPH Radical Scavenging Activity Inhibition (%) IC50, µg/mL | M. chamomilla Extract, µg/mL |
|---|---|
| 74.8 | 80 |
| 65.3 | 70 |
| 56.9 | 60 |
| 49.2 (IC50) | 50 |
| 41.29 | 40 |
| 32.4 | 30 |
| 27.5 | 25 |
| 22.15 | 20 |
| 18 | 15 |
| 5.8 | 10 |
4. Discussion
In our study, the extract of M. chamomilla significantly improved I/R-induced motor dysfunction. Experimental studies have shown that Matricaria chamomilla extract has various therapeutic effects such as immunomodulatory, anti-arthritic, sedative, anti-allergic, anti-bacterial and anti-oxidative, and can prevent cardiovascular disease and osteoporosis [17]. The effects of Matricaria chamomilla extract on the nervous system such as analgesic, anticonvulsant and memory-improving have been also reported [18-22].
The present study investigated the effect of ethyl alcohol extract of Matricaria chamomilla flower on cerebral ischemia-induced motor dysfunction in rats. Cerebral ischemia is reported to impair motor coordination and balance [23]. In the present study, Rotarod test was used to investigate the effect of cerebral ischemia and reperfusion on motor function. As expected, cerebral ischemia-reperfusion induced motor dysfunction in rats. Administration of ethyl alcohol extract of Chamomile following cerebral ischemia significantly improved motor in coordination. In Asgharzade et al. study, chamomile extract significantly ameliorated motor dysfunction induced by scopolamine [24].
A variety of mechanisms have been reported to be involved in the development of ischemic neuronal damage, including oxidative/nitrative stresses, DNA fragmentation and oxidation, increased production of inflammatory eicosanoids, increased activities of microglial and astrocytes, downregulation of anti-apoptotic proteins, and upregulation of proapoptotic proteins [25, 26].
Previous studies showed that transient cerebral ischemia induced lipid peroxidation and decreased the antioxidant capacity of the brain and serum [24, 27]. In our study, transient cerebral ischemia caused a significant increase in serum MDA level but did not affect the brain levels of MDA and TAC and the serum levels of TCA and NO. We observed that induction of transient cerebral ischemia did not cause a considerable oxidative stress in the serum and brain tissue of the rats. Therefore, motor dysfunction observed in our study may be related to the other pathways. It was reported that deterioration of motor function after ischemia is mainly due to disruption of the blood-spinal cord barrier and inflammation of both gray and white matters of brain [28].
According to the results, 50, 100, and 200 mg/kg of Matricaria chamomilla extract significantly reduced serum MDA level but did not change brain MDA levels. It had no significant effects on the total antioxidant capacity of the brain and serum. Injection of Matricaria chamomilla extract also did not change serum NO level. Therefore, the protective effect of M. chamomilla extract against ischemic brain damage may be related to the other mechanisms.
In Chandrashekhar et al. study, the methanol extract of German chamomile (Matricaria recutita L.) displayed considerable neuroprotective effects against cerebral ischemia through decreasing lipid peroxidation and increasing the superoxide dismutase, catalase, glutathione, and total thiol levels in the brain [29]. The inconsistency in the results our and Chandrashekhar et al.’ s study may be related to the species of the plant and the types of the solvent used.
The dried flower of Matricaria chamomilla contains terpenoids (α-bisabolol, α-bisabolol oxide, chamazulene, sesquiterpenes), coumarins (umbelliferone), flavonoids (luteolin, apigenin, quercetin), spiroethers (en-yndicycloether), tannins, anthemic acid, choline, polysaccharides, and phytoestrogens [30, 31]. The neuroprotective and therapeutic effects of M. chamomilla extract may be related to its bioactive components.
Quercetin has been reported to have anti-inflammatory, anti-coagulant, and anti-ischemic effects [32]. A study demonstrated that quercetin exerted inhibitory effect against ischemia and reperfusion-induced injury in various tissues including neural tissue [33].
A study by Ha et al. (2008) showed that apigenininhibited the production of NO by suppressing the expression of inducible NO synthase. It also protects the neuronal cells against injury in middle cerebral artery occlusion [34].
4.1. Conclusions
The Matricaria chamomilla extract was found to exert neuroprotective activity as it was evident from the reduction of lipid peroxidation and improvement of the motor coordination.