1. Background
Scaffold-based in vitro tissue engineering is a promising approach to designing artificial tissues for clinical purposes to overcome the limited availability of tissue donors and the possibility of viral contamination (1, 2). Natural scaffolds are frequently preferred for this purpose because of their biodegradability, microenvironment mimicking, improved cell adhesion, and establishment of homeostasis through proper immunological responses, mild-antigenic properties, and angiogenesis. These scaffolds are usually composed of biodegradable polymers, such as polysaccharides (e.g., cellulose, chitin, chitosan, alginate, dextran, xanthan, and hyaluronic acid), proteins and polyamides (e.g., collagen, gelatin, and silk), polyesters (polyhydroxyalkanoates family), and extracellular matrix (ECM) ingredients, including collagen, elastin, and fibronectin (3, 4). The type of scaffolds may affect their characteristics such as mechanical strength, natural microenvironment mimicking, tissue-compatibility, tissue growth, graft rejection, the interrelated porous network for cell nutrition, disposal of cellular wastes, ECM formation, porosity, pore size, and angiogenesis ability (5-7).
Chitosan, a deacetylated form of chitin, is a kind of degradable non-toxic biopolymers found in the exoskeletons of living organisms with a broad range of application in medical research such as wound and burn healing, bone break healing, surgical stitching, dentistry, drug delivery, contact lenses, pharmacy, coagulation factors, anti-cancer treatments, metal ions removal, and coagulators (protein, amino acid, and organic compounds) (8). Gelatin, a transparent and collagen-derived ECM component, is another suitable compound used for scaffold fabrication in several preclinical studies owing to its cellular cohesion capabilities (9). Chitosan and gelatin are opposite to each other in ionic nature and this characteristic facilitates their potential to use in scaffold formation. Gelatin is also of critical significance, as it maintains the ideal porosity for enhanced cellular cohesion and growth via reducing the nanofiber diameter and improving the surface area-to-volume ratio (10).
Chitosan and gelatin have been used to simulate in vivo environments in the form of 3D models to study the cell behavior in vitro as exhibited in live tissues. For this purpose, many experiments have been performed to improve the electrospinning of chitosan-gelatin nanofibers for artificial skin generation, wound recovery, and neuropathic injuries recovery (7, 10). Furthermore, studies seeking the ideal porosity of nanofibers have shown an ideal range of 100 - 150 µm for enhanced cellular support, growth, and migration (11, 12).
Despite the obvious differences between humans and animals, the rabbit’s pinna is a good experimental model for tissue reconstruction. Medical studies on biological and non-biological linking of cells to scaffolds have shown the feasibility of recovery and improvement of different tissue types. Blastema tissues are a group of undifferentiated cells with the ability to divide and differentiate in some parts of the living body. Reconstruction and recovery through stem cells occur in two steps: (1) transformation of mature cells to stem cells such as embryonic cells and (2) development of the cells into new tissues in the same manner they were initially formed (13). A good example of recovery in mammalians is the replacement of all tissues after generating a hole in the rabbit’s pinna. Through this process, blastema tissue (tissue model) is formed around the hole. Then, it replicates itself to form new cells on the site. These cells can generate cartilage, connective tissue, abdominal skin, back skin, and other parts entirely similar to the primary tissue in a few days (14, 15).
2. Objectives
The current study aimed to investigate the viability and migration of blastema stem cells (BSCs) cultured on the micro-nanofiber chitosan/gelatin scaffold (CGS).
3. Methods
3.1. Chemical
Gelatin and chitosan (Merck, Germany) were used for the preparation of scaffolds. Then, a powder was prepared composed of gelatin 35% (wt/v) and chitosan 5%. The powder was dissolved in acetic acid 50% (Merck, Germany) and distilled water as the solvent. The acidic and aqueous solutions were separately placed in a force-spinning device to fabricate nanofibers. Hence, to generate micro-nanofibers and prepare the electrospinning solution in 10 ml of acidic solvent 90% (v/v), we placed gelatin 7% (wt/v) and chitosan 7% (wt/v) in the electrospun device. Fibers were exposed to glutaraldehyde 25% (Merck, Germany) for 2 hours for stabilization. Then, a phosphate-buffered saline (PBS; DENAzist, Iran) solution was used for washing several times to remove the toxic effects of glutaraldehyde.
3.2. Scaffold Preparation
Forcespinning (FS) is a newly developed technology that uses the centrifugal force to spin nanofibers electrostatic force from polymer solutions. The forcespinning method (FSM) is performed in an electrical field using the centrifugal force (16, 17). The combination of the centrifugal force and different configurations facilitates the transformation to other states through the FSM. Some studies tried to find a new technique to remove, or at least, minimize some limitations to fiber fabrication in the electrospinning method. In addition to its simplicity and flexibility, such a system may allow for the mass production of fibers of polymers, metals, ceramics, and nanocomposites with diameters in the nanometer or micrometer scale with low costs.
3.3. Animals
Four male New Zealand white rabbits (2 - 3-months-old) with an average weight of 2,500 g were used in this study. The animals were maintained under standardized housing conditions (temperature 22 ± 2°C, 12-h light/dark cycle) in Plexiglas cages with free access to food (standard laboratory rodent chow) and tap water ad libitum.
3.4. Preparation of Blastema Tissue and Scaffold Placement
To obtain blastema tissues, the holes of 3 mm in diameter were created in the rabbit’s pinna using a puncher 11. Scaffolds composed of micro-nanofibers were mounted inside the tissue model rings in sterile conditions. The second holes (4 mm in diameter) were punched around the first ones on days 6, 9, and 15 to remove the blastema ring from the pinna. In this way, a scaffold with a cartilage ring or the scaffold alone was isolated and examined.
3.5. Light Microscopy
Scaffolds were first washed with PBS. Then, the sections of 7-µm thickness were stained using hematoxylin-eosin (H&E) and examined by a light microscope. Besides, some grafts were fixed dehydrated in a graded series of acetone and embedded in a resin, followed by staining by toluidine blue to examine under the light microscopy.
3.6. Scanning Electron Microscopy (SEM)
Scaffolds were fixed with glutaraldehyde, washed four times with Tyrocle’s cacodylate buffer (pH 7.4), dehydrated in a graded series of acetone, and coated with gold-palladium. Then, SEM images were taken by an SEM device (Leo VP 1450, Carl-Zeiss, Oberkochen, Germany).
4. Results
Investigating the interaction between the rabbit’s pinna blastema tissue and the chitosan-gelatin nanofiber scaffold confirmed the adhesion between cells and their penetration into the scaffold. Using gelatin not only improved the physical, chemical, and biological characteristics of chitosan, but also made it more flexible. Further, by reducing the diameter of nanofibers and increasing the surface area-to-volume ratio, it expanded the adhesion surface to the scaffold and enhanced the cell growth. In total, it can be concluded that chitosan and gelatin natural-synthetic nanofiber scaffolds provided a good basis for growth, migration, and differentiation of cells in the blastema tissue of rabbit’s pinna in vivo and in vitro. The SEM images of microfiber scaffolds prepared by the FSM are shown in Figure 1.
4.1. Images of Light Microscopy
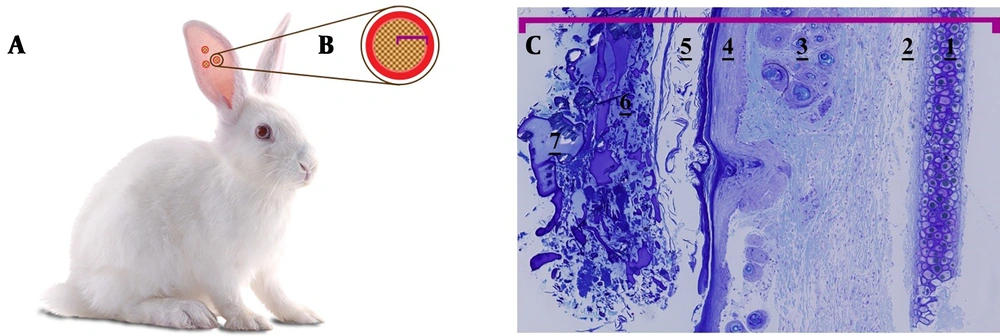
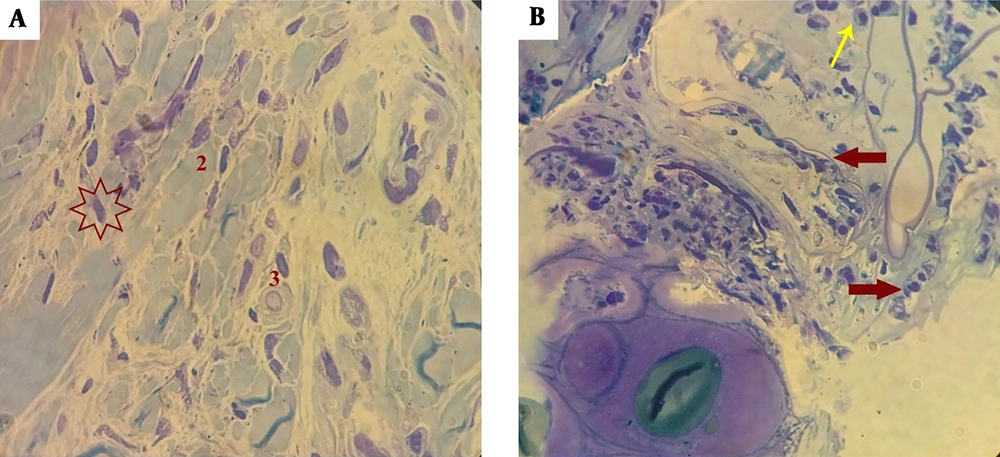
Cells integrated into scaffolds were placed in the blastema ring, isolated at 1, 6, 9, and 15-day intervals, and morphologically studied. It seemed that micro-nanofibers created by the FSM had a high adhesion strength to the blastemal ring and they survived well in the ear, as the results showed on days 1, 6, 9, and 15. Figure 2 presents a schematic view of the scaffold placement in the blastema ring of the rabbit’s pinna. Figure 2C displays the microscopic image of the blastemal ring after 6 days. Figure 3 shows the histological study of blastemal tissue on the 9th day after the placement of the scaffold in the rabbit’s eardrum. The elongation of the cells’ nuclei and their placement in a row, which is a kind of change in the dividing cells’ morphology, are shown in Figure 3B (red arrow).
The schematic view of rabbit eardrum blastema ring and optical microscope image of the blastema ring along with the scaffold on the 6th day (40× magnification). A, punched rabbit’s pinna; B, blastema ring and natural scaffold; C, outward-in: 1- cartilage, 2- connective tissue, 3- hair bulb, 4- epithelium, 5- ceratinin, 6 and 7- the microscopic content of the scaffold.
Histological examination of the scaffold’s blastema cells on the 9th day (1000× magnification). A, 1- cytoplasm wastes on blastema cells on day 9; 2- concentrated and differentiated nuclei of the blasts; 3- clear undifferentiated nuclei; B, orientation of blastema cells in the scaffold (thick arrow); a number of inflammatory cells (thin arrows).
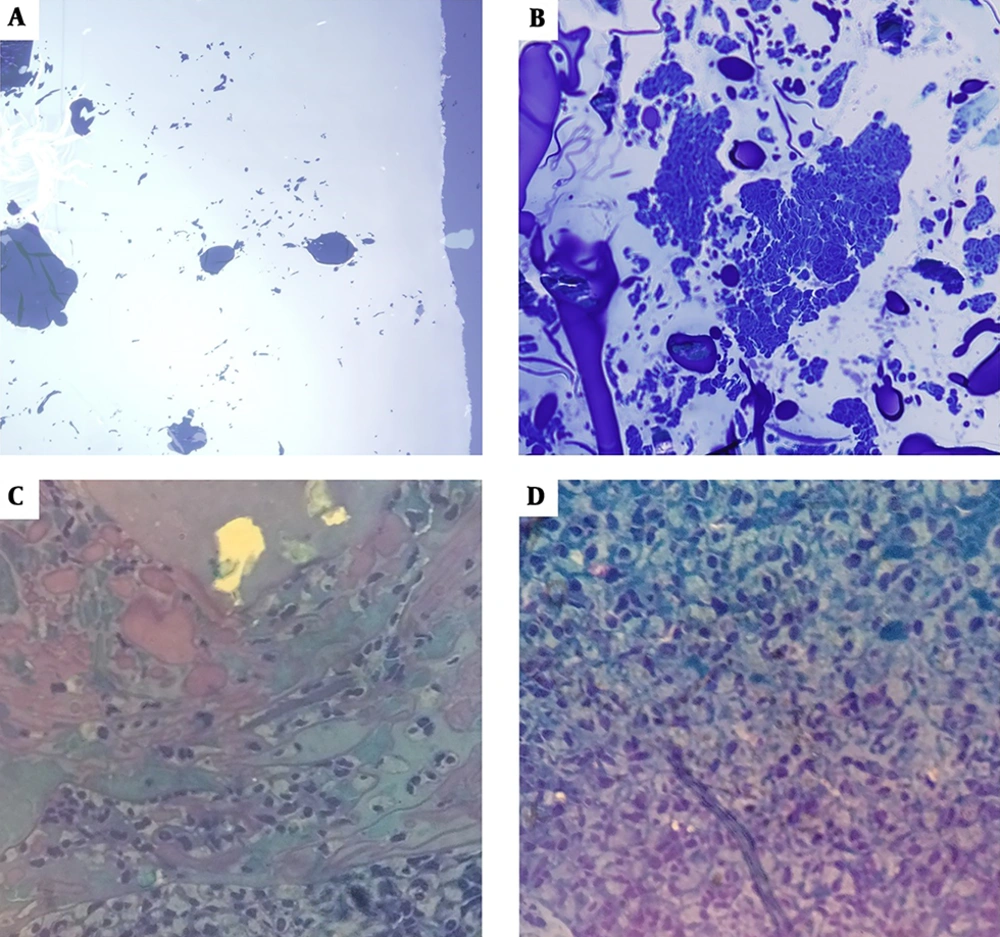
Tissue investigation on the first day showed no permeation and migration (Figure 4A). On the sixth day, cells permeated peripheral regions and concentrated on the middle parts of the scaffold (Figure 4B). On the ninth day, a considerable number of cells were aligned in the same direction and showed the same morphology with extended cores (Figure 4C). On the 15th day, a reduction in immune cells was observed and a significant increase in blastema cells was evident. The 15th day was recognized as the optimal day of the procedure because of the higher density of cells permeated the scaffold. Moreover, cells with an extended morphology and a spindle-shaped core (morphologically similar to fibroblast cells) were observed throughout the scaffold (Figure 4D).
Light microscope images of blastema cells on nanofiber CGS (1000× magnitude). A, CGS on day 1 (lacking any cells); B, CGS on day 6, arrow tip shows the accumulation of a huge number of blastema cells on the scaffold; c) the scaffold on day 9 with many immune system cells (leukocytes) around (*); cells are dispersed all around the matrix (arrow tip); D, CGS on day 15: the number of blastema cells increased and they turned to spherical shapes (arrow tip), the number of leukocytes was reduced (*).
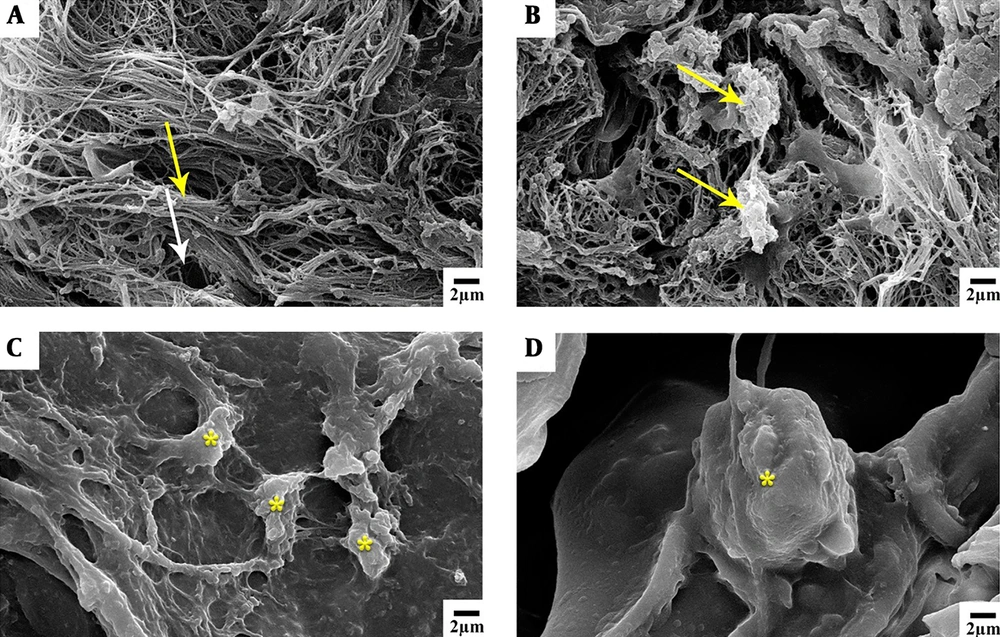
4.2. SEM Images
The SEM images of the scaffold taken one day and nine days post-implantation are shown in Figure 5. The cohesion and extension of cells on the scaffold on the ninth day are obvious.
5. Discussion
Most cells of the mammalian species require junctions to develop growth and proliferation. Therefore, if the appropriate substrate is not provided for the adhesion of the cells, they will demonstrate (18). The chitosan-gelatin scaffold provides a suitable environment for the induction of cell adhesion, proliferation, and migration. It seems that the chitosan-gelatin scaffold is a more appropriate option for tissue engineering and stem cell transplantation than the pure chitosan scaffold (19). The results of the present study confirmed this function in blastema tissue. The histological examination showed that none of the cells migrated to the scaffold on the first day of the scaffold implantation inside the blastema ring. On the sixth day, cells penetrated the scaffold, but accumulated merely in a section of the scaffold. On the ninth day, cells were proliferated in all parts of the scaffold. The peak of cells penetration into the scaffold occurred on the 15th day after implantation. At this time, the cells morphologically altered. These findings confirm the increased adhesion, migration, and differentiation of blastema cells on the scaffold owing to preparing a 3D structure and suitable microenvironment for the cells (15).
Chitosan is a natural compound that makes a biodegradable, biocompatible, and nontoxic scaffold. After being electrospun in the form of nanofibers, chitosan shows promising potential in tissue engineering for recovering wounds and bone/cartilage injuries (8, 12). Scaffold-based tissue engineering approaches have been applied to generate 3D models for studying cell behaviors (morphology, replication, migration, differentiation, and survival) and interactions with scaffolds (15). Chemical composition and physical properties of natural ECM have significant impacts on morphology, survival, and migration of cells. In addition, ECM directly interacts with cell skeleton, migration, differentiation, and survival. The elasticity of ECM may also have an important role in the behavior of cells in terms of migration, death, and replication (15, 20).
A previous study showed the migration and cohesion of blastema cells to the decellularized gullet scaffold and implied the important role of nanofiber CGS in migration, cohesion, and replication of blastema cells of the rabbit’s pinna (15). Blastema cells are very similar to stem cells with high proliferation and differentiation potential (11). The migration of blastema cells in 6 days to the scaffold confers that materials used in CGS have developed a natural substrate for cell migration. However, the scaffold loses its structure after two months and disintegrates in the body because of its biocompatibility (11). One reason behind the better condition for blastema cells migration to the scaffold may be the pore sizes while comparing the diameter of pores and spaces between micro-nanofibers and nanofibers generated by forcespinning. It has been shown previously that amnion membrane epithelial cells have a more chance to connect to gelatin and chitosan beds in larger pore sizes (11). In rabbit, the normal time required for the hole closing is around 1-2 months. The migration of cells to the scaffold and the cellular accumulation in day 6, dispersal in day 9, and replication in day 15 may indicate the inductive properties of the scaffold in reconstructing the tissue and as a result, the increased growth and replication of blastema cells for rapid reconstruction. The results of previous studies indicate that chitosan, as a biodegradable, biocompatible, nontoxic polymer, not only induces growth and proliferation of bone marrow stromal cells and skin fibroblasts (10), but also acts as a drug carrier biopolymer (21). It also enables the cells to build a neural conduction channel for repairing the peripheral nervous lesions (7). Based on the results of the present study, it seems that chitosan in the structure of the scaffold stimulated blastema cells for better growth and proliferation. The results of the histological evaluation indicated an increase in the number of cells and a change in the shape of their nuclei from round to stretched shapes.
The presence of gelatin in the structure of the scaffold increases the surface area for better connection of cells to the scaffold and thus, increasing the extent of adhesion and growth of the cells on the scaffold (10). The results of this study showed that the natural nanofiber scaffolds made of chitosan and gelatin were suitable substrates for growth, migration, and differentiation of rabbit’s ear canal blastema cells in vivo and in vitro.
5.1. Conclusions
According to the findings, it seems that natural nanofiber CGS provides a proper substrate for the growth and migration of blastema cells. However, more research is needed to confirm these findings. It is suggested that the inductive effects of natural-synthetic scaffolds and their interaction with stem cells be further studied.