1. Background
Excessive intra-operative bleeding during orthopedic surgeries is a serious condition which is accompanied by some risks. For example, infection after surgery with the amount of bleeding and postoperative anemia can cause a delay in patient discharge from the hospital and affect the patient's performance (1, 2). Blood transfusions have always been associated with complications such as injection response and increased risk of infection. Therefore, researchers and anesthetic specialists have always been looking for ways to reduce the amount of bleeding during the operation. So far, many methods have been tried to achieve this goal; among them are hemodilution, the use of hypotension (reducing blood pressure), and the application of antifibrinolytic drugs (3-6).
When a high rate of bleeding occurs during the operation, it is managed by colloidal fluids and blood transfusion. Plasma fibrinogen is reduced sooner than any other coagulants, which can lead to broader bleeding that requires a blood transfusion. Fibrinogen (factor 1) is a critical coagulation factor in the formation of clot and platelet aggregation by binding to the IIb/IIIa glycoprotein. Due to this crucial role, fibrinogen has been used successfully during cardiac surgery, which has a high bleeding rate and may not respond appropriately to other cases (7-11).
As the hip and acetabulum surgeries are associated with high bleeding possibility, the use of prophylactic fibrinogen may be useful in bleeding reduction (12-19).
2. Objectives
The lack of a standard method for controlling bleeding during hip surgery; and useful effectiveness of fibrinogen during cardiac surgery resulted in this study aimed at the investigation of fibrinogen on the bleeding level during hip surgery.
3. Methods
This double-blinded randomized clinical trial was conducted in Firoozgar Hospital from July to December 2019 and was registered by Iran Clinical Trials.gov (IRCT20200109046064N1) before recruitment. The study protocol was approved by the Ethics Committee of Iran University of Medical Sciences, and all the patients were fully informed before they signed a written consent form before registration.
The average amount of bleeding in those undergoing hip replacement surgery is about 1,200 mL, and the standard deviation is approximately 300 mL (20). We expect this amount of bleeding to be reduced to 900 mL by administering fibrinogen before surgery (20). Considering α = 0.05 and β = 0.1, the sample volume required to show the 300 mL difference between the two groups is calculated from the following formula:
2N = ∑ * (Z1 - α/2 + Z1 - β)2 * σ2/δ2; σ = 300 cc, δ = 300 cc, 2N = 42.
A total of 42 patients scheduled for non-emergency pelvic surgery with a preoperative fibrinogen level of 2 - 4 g/L,18 - 60, and BMI of less than 30 kg/m2 were included. In contrast, patients having complications such as respiratory infection, cardiovascular disease, liver or renal disease and coagulation disorders, hypertension, diabetes mellitus, those taking beta-blockers, calcium-blockers, digoxin, a tricyclic antidepressant, anti-coagulant and clonidine, a history of alcohol and drug abuse, or tumor in the area of surgery were excluded.
The patients were randomly divided into two groups of fibrinogen and control using a blocked randomization method (block of 4).
Hemoglobin, platelets, coagulation tests, and fibrinogen levels were measured in all patients before surgery. The usual rate of plasma fibrinogen was 2 to 4 g/L. In order to prevent potential complications of hyper coagulopathy resulting from the prophylactic infusion of fibrinogen, just for patients with preoperative fibrinogen level ≤ 4 one-gram fibrinogen in 50 mL normal saline over a 15-minute period was slowly infused after the induction of anesthesia. In the control group, the patients received the same volume of normal saline instead. For blinding and hiding the samples, a special code was written for each patient inside the enclosed envelope and was only opened by the researcher confidentially before anesthetizing the patient. The results of changes and bleeding rates, and other components of this plan were recorded by a non-informed person. Pulse oximeter and heart rate were determined and recorded for all patients in the operating room after connecting monitoring NIBP.
In both groups in the operating room, a peripheral IV line was taken for each patient, and routine monitoring was performed. The patients received 5 mL/kg of normal saline before anesthesia induction. For pre-medication, they received Midazolam 25 mg/kg and fentanyl 3 mg/kg. Induction anesthesia was performed with propofol 2 mg/kg and atracurium 0.5 mg/kg. The maintenance of anesthesia was done using propofol 100 µg/kg/minute, atracurium 10 µg/kg/minute, and morphine 0.1 mg/kg. Nitroglycerin 2 to 10 mg/minute was also added.
Mechanical ventilation with these specifications was used: Tidal volume 8 mL/kg, respiratory rate 12 breaths per minute, PMax 35 cm H2O, 1:2 inhale to exhale ratio, and in all patients using capnography, ETCO2 was monitored about 35 mmHg. The volume of hemorrhage was calculated based on the bleeding collected in the suction and the gases soaked in the blood that had already been weighed. It should be noted that in the case of hypotension, the dose of TNG was reduced first, then the dose of anesthesia was modified; afterward, the blood pressure was controlled using some fluids during the operation. To control blood pressure and to provide relative controlled hypotension (mean blood pressure between 65 - 85), TNG 5 - 20 µgr/minute was used. Indications of blood transfusion were based on hemoglobin before the operation and calculating the patient's allowable blood loss (abl) (21).
Coagulation tests include an international normalized ratio (INR), prothrombin time (PT), partial thromboplastin time (PTT), and platelet count.
All data about bleeding amount, transfused blood, blood pressure, duration of the surgery, hemoglobin, platelet, and fibrinogen level were recorded during the first 24 hours after surgery in the data collection form.
Finally, 42 patients that were divided into 2 groups with the methods described entered the study.
3.1. Statistical Analysis
All the study data were analyzed using SPSS V22. First, the normality of quantitative variables was assessed based on Kolmogorov-Smirnov test and was confirmed. Therefore, in order to compare quantitative variables in two groups, t-independent test was used, and to compare qualitative variables in two groups, chi-square or Fisher exact test was applied. In addition, to compare quantitative variables in 2 groups before and after the surgery, t_ paired was used, and P < 0.05 was considered significant.
4. Results
In this study, 42 patients were referred to the operating room of Firoozgar Hospital for pelvic surgery in 2019 and required general anesthesia. Twenty-one patients were in the fibrinogen group, and 21 patients were in the placebo group.
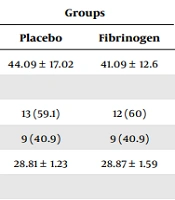
There were no significant differences between the groups regarding the age, sex, BMI, ASA, comorbidities, type of surgery, international normalized ratio, prothrombin time, partial thromboplastin time, blood transfusion, and platelets of patients (P > 0.05) (Table 1).
| Variables | Groups | P Value | |
|---|---|---|---|
| Placebo | Fibrinogen | ||
| Age (y) | 44.09 ± 17.02 | 41.09 ± 12.6 | 0.588 |
| Sex | 0.92 | ||
| Female | 13 (59.1) | 12 (60) | |
| Male | 9 (40.9) | 9 (40.9) | |
| BMI (kg/m2) | 28.81 ± 1.23 | 28.87 ± 1.59 | 0.897 |
| Comorbid disease | 0.352 | ||
| No | 8 (36.4) | 14 (70) | |
| Diabetic | 3 13.6) | 2 (10) | |
| Hypertension | 6 (27.3) | 2 (10) | |
| Smoke | 3 (13.6) | 1 (5) | |
| Hyperlipidemia | 2 (9.1) | 1 (5) | |
| Type of surgery | 0.745 | ||
| Acetabulum | 6 (27.3) | 4 (20) | |
| Hip fracture | 2 (9.1) | 2 (10) | |
| Total hip | 10 (45.4) | 8 (40) | |
| Periacetabular osteotomy | 4 (18.2) | 6 (30) | |
| INR | 1.07 ± 0.09 | 1.05 ± 0.11 | 0.245 |
| PT | 12.7 ± 0.59 | 13.3 ± 1.26 | 0.059 |
| PTT | 32.14 ± 4.17 | 29.66 ± 3.97 | 0.056 |
| Platelets (gr/L) | 253.09 ± 36.64 | 213.95 ± 38.9 | 0.062 |
Comparison of Age, Sex, BMI, ASA, Comorbid Disease, Type of Surgery, and Parameters of Preoperative Coagulation of Patients in the Two Groups a
There was no significant difference between blood transfusion in the two groups (P = 0.175), but total bleeding was significantly lower in the fibrinogen group than in the control group (P = 0.022) (Table 2).
| Variables | Groups a | P Value | |
|---|---|---|---|
| Placebo | Fibrinogen | ||
| Bleeding (mL) | 1519.5 ± 289.4 | 1328.57 ± 227.8 | 0.022 |
| Transfusion (bag) | 1.09 ± 0.76 | 0.8 ± 0.51 | 0.175 |
Comparison of Bleeding and Transfusion of Patients in the Two Groups
There were no significant differences regarding the pre-operative and postoperative hemoglobin and serum fibrinogen between the two groups (P > 0.05) (Table 3).
| Variables and Time | Groups | P Value b | |
|---|---|---|---|
| Placebo | Fibrinogen | ||
| Hemoglobin (gr/dL) | |||
| Before surgery | 13.71 ± 1.63 | 14.1 ± 1.3 | 0.318 |
| After surgery | 13.74 ± 1.62 | 14.08 ± 1.33 | 0.338 |
| P value c | 0.825 | 0.898 | |
| Serum fibrinogen (mg/dL) | |||
| Before surgery | 353.09 ± 22.27 | 356.42 ± 30.41 | 0.462 |
| After surgery | 352.61 ± 22.45 | 354.52 ± 31.34 | 0.527 |
| P value c | 0.682 | 0.059 | |
Comparison of Hemoglobin and Serum Fibrinogen During Surgery of Patients in the Two Groups a
5. Discussion
The present study examined the effect of prophylactic fibrinogen injection on perioperative bleeding during pelvic surgeries. Administration of fibrinogen in this study, although significantly decreased bleeding, did not affect blood transfusion. Previous studies on other surgeries have also shown similar evidence on the amount of bleeding. Several types of research confirm that the plasma concentrations of fibrinogen at the time of surgery determine the amount of bleeding and the need for a blood transfusion (22-24). Ucar et al. found that on 97 patients with CABG, the high level of fibrinogen was consistent with the amount of chest tube discharge 48 hours after surgery (23). In the study done by Karlsson et al., similar to the present study, fibrinogen concentrate infusion reduced postoperative blood loss by 32% (22). Of course, in some studies such as Shibata and his colleagues, which was performed on women with postpartum hemorrhage within the first 24 hours after delivery, the early transfusion of fresh frozen plasma (FFP) only reduced bleeding in women with low fibrinogen levels. This is very important concerning the significant role of fibrinogen in homeostasis, especially in pregnant women who are exposed to coagulation disorders and the risk of bleeding and death, and necessitates its administration (25). This issue was also dealt precisely with by Charbit’s et al., which indicated that a simple fibrinogen measurement could anticipate the risk of severe bleeding in postpartum hemorrhage (26). Also, in the study of Rourke and colleagues, which was performed on 517 traumatic patients, the amount of bleeding in these patients was inversely correlated with blood fibrinogen level and predicted the need for blood transfusion. Also, in this study, fibrinogen level was able to predict the mortality rate of 24 hours and 28 days (27). Likewise, Pournajafian et al. found that the administration of prophylactic fibrinogen in posterior spinal fusion surgery significantly reduced the amount of blood transfusion (19). In the study conducted by Janatmakan et al., it was cited that the administration of fibrinogen in patients with a high risk of bleeding may decrease the amount of bleeding and the need for subsequent blood transfusion after open prostatectomy surgery. There were no significant differences regarding hemoglobin level and blood fibrinogen level between the patients receiving fibrinogen and the control group (28).
As mentioned above, in all of the studies cited, in addition to reducing the amount of bleeding, the blood transfusion rate was significantly reduced after fibrinogen administration, which was different in the current study. However, in a survey conducted by Wafaisade et al., performed on 294 traumatic patients, the early use of fibrinogen administration was associated with significantly lower 6-hour mortality and an increased time to death, but the reduction of overall hospital mortality was not observed. Also, in his study, the same as the current one, it was found no difference in the amount of blood administration needed (29). Another point that was different in our study was that prescribing prophylactic fibrinogen was done in patients with average fibrinogen levels, whereas previous studies specified fibrinogen was limited to patients whose fibrinogen levels were lower than normal levels.
5.1. Conclusions
According to the findings of previous studies as well as the present study, it seems that injection of prophylactic fibrinogen to patients with normal fibrinogen levels in pelvic surgeries significantly reduces bleeding rates but does not decrease the rate of blood transfusions to these patients, which requires further studies.